BACKGROUND
Tyrosine Kinase Inhibitor (TKIs) discontinuation has become, nowadays, under proper conditions, a feasible option for chronic myeloid leukemia (CML) also in "real life" setting. Different papers investigated which factors (age, sex, type of TKI, previous r-interferonα -r-INFα- therapy, line of therapy at stop, type of transcript, duration of TKI therapy and of sustained deep molecular response -sDMR-, Sokal risk score) could predict a successful TKIs discontinuation either within protocols or outside of clinical trials, and the results are not unique.
AIM
We retrospectively evaluated our CML pts who stopped TKI after sDMR in order to assess the variables that could influence the probability of a durable TFR.
METHODS
BCR-ABL transcripts were determined by RQ-PCR performed according to EAC protocol (Gabert et al, 2003) and to the standards of the Italian National Network Labnet. Criteria for TKI discontinuation was sustained DMR (MR4 or better) for at least 2 years. After TKI withdrawal, RQ-PCR for BCR-ABL was performed every month during the first year and every 2 months thereafter. TKI treatment was reintroduced if DMR loss occurred.TFR was assessed using the Kaplan-Meier method; potential prognostic factors were considered for multivariable analyses at a level less than .20.
RESULTS
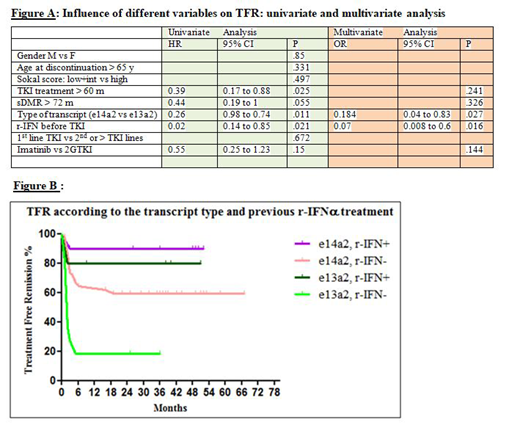
Between October 2010 and January 2019, 68 patients discontinued TKIs, 18 of them after less than 5 years of treatment because of pregnancy desire (3), intolerance (6), patient's desire/non compliance (5), enrollment in study protocols (4). At discontinuation median age was 63 years (30-85), median time from TKI start 85 months (30-190), median duration of sustained DMR 48 months (24-153). Sokal distribution was 48%, 31% and 18% for low, intermediate and high risk respectively (2 patient was not evaluable). E14a2 transcript type was present in 52 pts and e13a2 in 16 pts. Thirty-eight patients stopped imatinib, 25 nilotinib (19 in 1st line, 6 in 2nd line), 5 dasatinib. Before imatinib 15 patients received r-IFNα, for a median time of 60 months (3-256). Median follow up after TKI stop was 39 months (5-105, >24 in 61, <12 in only 2 patients). Twenty-eight (41%) patients lost DMR. Median time off-therapy for these patients was 3 months (1-19), only 2 lost DMR after 6 months (at +16 and +19 months). One patient aged 87 years has not yet resumed therapy but remains in stable MR3 at 34 months after TKI discontinuation. Therapy was restarted in 27 patients (1 in MR1, 11 in MR2, 15 in MR3), 24 achieved a second DMR after a median interval of 2 months (1-18); 3/27 patients are in M3 after 2, 22 and 26 months. Neither cytogenetic relapses, nor progressions were documented. One patient died in DMR for pancreatic cancer. Univariate analysis showed no difference in relapse risk according to age, gender, type of TKI (imatinib vs 2nd generation TKIs), and Sokal score, while the e14a2 vs e13a2 transcript type (p = 0.011), duration of TKI therapy > 60 months (p = 0.025) and previous r-IFNα therapy (p=0.021) were significantly linked to better outcome after TKI discontinuation; sDMR > 72 months is very close to be a significant variable (p=0.055). At multivariate analysis only the type of BCR-ABL transcript (p=0.027) and previous r-IFNα ( p=0.016) remained independent significant prognostic factors -figure A and figure B-.
CONCLUSION
e14a2 transcript type was confirmed as a robust favorable prognostic factor for TFR maintenance. In our experience, 2GTKIs didn't impact favorably TFR duration after TKIs discontinuation, conversely r-IFNα treatment before TKI improved the probability of maintaining DMR after TKI withdrawal, particularly in e13a2 patients. In fact r-IFNα before imatinib reversed the negative prognostic impact on TFR maintenance of the e13a2 transcript type.
D'Adda:Novartis: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Rossi:Amgen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; BMS: Honoraria; Sandoz: Honoraria; Jazz: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Daiichi-Sankyo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal