Background. Cardiovascular adverse events (CV-AE) are emerging complications in chronic myeloid leukemia (CML) patients treated with second and third generation tyrosine kinase inhibitors (TKIs). Despite the importance of CV risk factors,predictive CV-AE biomarkers are still lacking. Further understanding of the molecular pathways underlying CV-AE may promote novel strategies to prevent its initiation prior to clinical disease. In this scenario, the use of a novel tool such as metabolomics may be useful for the identification of new metabolic pathways related to CV-AE. Metabolites are the output of cellular metabolism, accounting for expression and activity of genes, transcripts, and proteins, and offering unique insights into small molecule regulation. For the first time we evaluated the correlation between CV-AE and metabolomic profile in CML patients treated with TKIs.
Methods. We considered 39 adult CP-CML patients (mean age 49, range 24-70), without comorbidity at baseline, consecutively diagnosed and treated with imatinib, dasatinib nilotinib and ponatinib, at the Haematology Unit of "Businco Hospital", Cagliari, Italy. All patients underwent a metabolomic profile detection, after CV-AE or during follow-up, and were stratified in 2 groups (with or without CV-AE). Plasma samples were collected and acquired chromatogram was analysed by means of the free software AMDIS (Automated Mass Spectral Deconvolution and Identification System; http://chemdata.nist.gov/mass-spc/amdis) that identified each peak by comparison of the relative mass spectra and the retention times with those stored in an in-house made library comprising 255 metabolites. Data were investigated by applying the supervised multivariate statistical approach OPLS-DA (Orthogonal partial least square discriminant analysis) (SIMCA, version 13.0, Umetrics, Umea, Sweden).
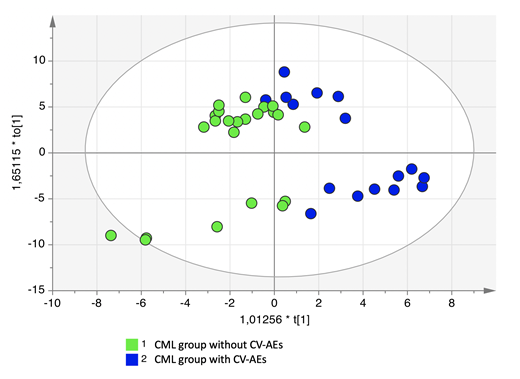
Results. The mean follow-up since CML diagnosis was 3.7 years (range 0.9-5); 22 (56.4%) patients were treated frontline, while 17 (43.5%) underwent second or subsequent TKI lines of treatments. The reason for switching was inefficacy in 15.3% and intolerance in 28.2%. At CV-AE or last follow-up 16 (41%) patients were treated with imatinib, 8 (20.5%) with dasatinib, 14 (35.8%) with nilotinib and 1 patient with ponatinib (2.7%). Overall, 17 CV-AE were recorded: 7 cases of hypercholesterolemia, 5 pleural or pericardial effusions, one episode of hypertension and 4 cardiac events (atrial fibrillation,ST-segment elevation myocardial infarction, reduction of cardiac ejection fraction and dissecting aneurysm of the aorta); 7 CV-AE were graded as 3 according to the common toxicity criteria and one patient died from dissecting aneurysm of the aorta). The 60-month cumulative CV-AE incidence was 54.4±9.1%. The mean time between the start of the treatment and the occurrence of a CV-AE was 44.4 months (range 19-60). OPLS-DA showed that patient's samples were clearly separated into 2 groups indicating that CV-AE patients (blue dots) presented a markedly distinct metabolic profile compared with patients without CV-AE (green dots); (figure 1). The parameters of the model were R2Y = 0.76 and Q2 = 0.44. To validate the OPLS-DA model, a permutation was performed resulting statistically significant (p=0,002). The main discriminant metabolites were tyrosine, lysine, ornithine, glutamic acid, 2-piperdincarboxylic acid, proline, citric acid, phenylalanine, mannitol, threonine, leucine, creatine, serine, 4-hydroxyproline, and alanine (more represented in CV-AE group); while unknown 204, myristic acid, arabitol, oxalic acid, 4-deoxyrithronic acid, elaidic acid and ribose resulted less expressed in CV-AE group.
Conclusions. This exploratory study showed different metabolomic profile of CML patients with CV-AE underwent TKI treatment, suggesting possible mechanisms of endothelial damage mediated by the accumulation of metabolites. Tyrosine, highly expressed in the CV-AE CML group, is a reliable marker of oxidative stress in various acute and chronic diseases.Metabolomics research has considerable potential for translating the metabolic fingerprint into personalized therapeutic strategies. These preliminary data should be confirmed in prospective clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal