Background: In February 2017, CT-P10 became the first rituximab biosimilar to be approved in Europe for treatment of rheumatic diseases and specific blood cancers including non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukaemia (CLL). More recently, CT-P10 was approved in the US for NHL. Although the recommended rituximab administration protocol is a slow initial infusion rate with gradual up-titration, rapid infusion protocols are used widely in patients with no serious complications from their first infusion. There are limited data on the safety of rapidly infused CT-P10.
Aims: To evaluate the safety and effectiveness of rapidly infused CT-P10 in patients with NHL or CLL in a real world clinical setting over a 6 month follow up period.
Methods: This non-interventional post-authorisation safety study is in progress in the United Kingdom (UK), Spain, France and Italy. It involves collection of data from the medical records of consenting adult patients with NHL or CLL who received rapidly-infused CT-P10 (total infusion time ≤90 minutes) during routine clinical practice. The index date (day 1) is the date of the first rapid CT-P10 infusion. Safety and effectiveness data have been collected over a 6 month observation period from the index date (or to death, if sooner). Early results from this study have been previously presented and showed the index IRR rate (the primary outcome) to be 8% (Bishton, 2019). Updated interim results with full 6 month follow-up are now available for 112 patients and are reported here, based on a data cut on 19 June 2019.
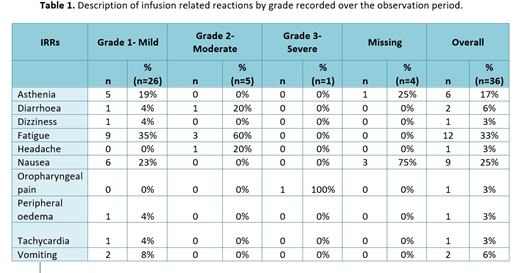
Results: This interim analysis includes patients enrolled from the UK, Italy and Spain. Ninety four patients (84%) have NHL (68 [61%] diffuse large B-cell lymphoma [DLBCL], 26 [23%] follicular lymphoma [FL]) and 18 (16%) have CLL. Other patient characteristics at index date: 74 (66%) male; median age 67 years (interquartile range [IQR] 58.0-74.0); median disease duration 0.2 years (IQR 0.1-0.4); 20 (18%) patients with prior treatment recorded (n=111); Ann Arbor stage for NHL at index (n=58): I (n=7, 12%), II (n=7, 12%), III (n=8, 14%), IV (n=35, 60%) or other (n=1, 2%; recorded as '1E'); Binet stage for CLL at index (n=11): A (n=5, 45%), B (n=4, 36%) or C (n=2, 18%). Patients had between 1 and 4 CT-P10 infusions prior to the index date, with first infusions given over a median of 4 hours (IQR 3.5-4.1 hours). Median CT-P10 dose at index was 375 mg/m2 (IQR 375.0-375.0 mg/m2) and a median of 5 infusions per patient (IQR 3.8-6.0) were given over 6 months. A total of 512 CT-P10 infusions were given over the 6 month observation period across all 112 patients. Of 19 (17%) patients who experienced one or more IRRs over the observation period (including index IRRs), 9 patients had one, 5 had two and 5 had three or more IRRs. Thirty six IRRs were reported in total and are listed in Table 1 by grade. Ninety six (86%) patients experienced an adverse event (AE) and 31 (28%) experienced a serious AE (SAE) over the observation period. At 6 months post-index, 95 (85%) patients had discontinued first line CT-P10 due to planned completion of their treatment course, 7 (6%) had discontinued due to AEs, 1 (1%) due to disease progression and 5 (4%) due to other reasons (reason for discontinuation not known for 1 patient [1%]), with treatment ongoing for 3 patients (3%). Best responses to CT-P10 during the observation period (as documented by the local investigator) were complete response (n=83, 74%), partial response (n=24, 21%), stable disease (n=3, 3%) and progressive disease (n=2, 2%).
Summary/conclusions: In this multi-country study of patients treated with rapidly-infused CT-P10 in a real world setting, a high proportion of patients achieved complete or partial responses over 6 months follow-up. The results also suggest that CT-P10 is generally well-tolerated, with 36 (predominantly mild) IRRs recorded over a total of 512 infusions, and the majority of patients discontinuing CT-P10 due to planned completion of treatment.
Bishton:Takeda: Other: Travel support, Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel support, Research Funding; AbbVie: Research Funding; Celltrion: Membership on an entity's Board of Directors or advisory committees, Other: travel support. Zinzani:Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kim:Celltrion: Employment. Nam Lee:Celltrion: Employment.
CT-P10 is a rituximab biosimilar, approved in Europe for treatment of rheumatic diseases and specific blood cancers including non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukaemia (CLL). In Europe, the recommended administration protocol is a slow initial infusion rate with gradual up-titration. However, rapid infusion protocols are used routinely in many countries for second and subsequent infusions in patients with no serious complications from their first infusion. There are limited data on the safety of rapid infusion of CT-P10 so this non-interventional study aims to address this gap and reflect practice in the real world. Rapid infusion is on-label in the United States.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal