<Background> Serum soluble interleukin-2 receptor (sIL2R) levels are often measured to evaluate the state of lymphoma. The serum sIL2R level at diagnosis has been reported to be correlated with the prognosis of diffuse large B cell lymphoma (DLBCL) patients treated with the R-CHOP regimen. However, it is unclear whether interim sIL2R levels are associated with prognosis in DLBCL. Here, we analyzed the prognostic impact of interim serum sIL2R levels in DLBCL.
<Patients and Methods> We retrospectively examined data for DLBCL patients who started receiving chemotherapy at the Japanese Red Cross Society Kyoto Daini Hospital between January 2012 and December 2018. All of the patients received R-CHOP-like regimens (rituximab plus pirarubicin or adriamycin, cyclophosphamide, vincristine, and prednisolone). The interim sIL2R level (I-IL2R) was defined as the value measured after the third chemotherapy cycle. I-IL2R levels of >700 U/ml were regarded as positive. The primary endpoints of this study were progression-free survival (PFS) and overall survival (OS). The unadjusted probabilities of PFS and OS were estimated using the Kaplan-Meier method. The log-rank test and multivariate Cox regression analysis were used to assess the prognostic value of each clinical variable.
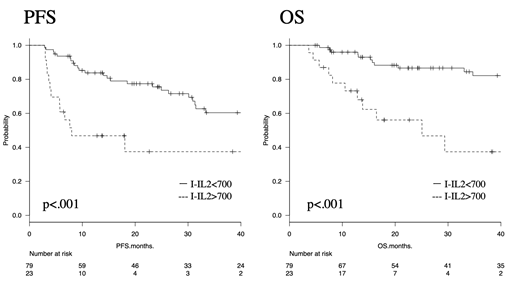
<Results> In total, 102 patients were enrolled. The patients' median age was 73.5 years (range, 35-88), 58 patients (56.9%) were male, and 52 (51.0%) had poor revised International Prognostic Index scores. The median follow-up time was 25.2 months (range, 3.7-88.6). Twenty-three patients (22.5%) were I-IL2R-positive (>700 U/ml). Univariate analysis revealed that I-IL2R-positivity was associated with a poor prognosis. The 3-y PFS rates of the I-IL2R-negative (<700 U/ml) and I- IL2R-positive (>700 U/ml) patients were 60.4% (95% confidence interval [95%CI], 46.2-71.9) and 37.5% (95%CI, 15.7-59.4; p<0.001, log-rank test), respectively, and their 3-y OS rates were 82.2% (95%CI, 69.7-89.9) and 37.4% (95%CI, 13.8-61.4; p<0.001, log-rank test), respectively. Multivariate analysis confirmed that the I-IL2R level is independently associated with prognosis.
<Conclusion> The I-IL2R level of >700 U/ml patients had poor prognosis. The I-IL2R level can be used to predict the outcomes of DLBCL patients. IL2R levels should be measured during chemotherapy, and I-IL2R-positive patients could be targeted with high-dose or novel therapies. As this study was based on a retrospective analysis and involved a small cohort and a limited follow-up period, further studies are needed to confirm the prognostic impact of I-IL2R.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal