Introduction:
The use of PET-CR as a surrogate endpoint would expedite the development of novel therapies and enable better estimates of sample size based on early outcomes of a trial. Previous studies have reported an association between end-of-therapy (EOT) PET results and long-term progression-free (PFS) and overall survival (OS) in diffuse large B-cell lymphoma (DLBCL) patients receiving standard first-line chemoimmunotherapy. We have also previously shown that the overall predictability of PET-CR for PFS and OS in these trials is similar to that in 18 literature-based studies. (15-ICML 2019, P195). To assess the potential for PET-CR as a surrogate endpoint in registration trials, we conducted a prospectively designed individual patient-level-data meta-analysis of available clinical trials.
Methods:
We synthesized patient-level data from three prospective phase II and III trials (GOYA [NCT01287741], GATHER [NCT01414855], MAYO [NCT00670358]) conducted in previously untreated DLBCL patients, using a Bayesian hierarchical model. We considered the two treatment arms in GOYA (GOYA-R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone] and GOYA-G-CHOP [obinutuzumab, cyclophosphamide, doxorubicin, vincristine, and prednisone]) separate; hence a total of four arms, excluding patients without baseline PET scans. We investigated the relationship between PET-CR and long-term survival outcomes overall and within patient subgroups. We used Kaplan-Meier plots to compare survival endpoints by PET-CR status. We considered hypothetical RCTs that have PET-CR and PFS as endpoints to show how our model of the relationship between PET-CR and PFS can be used to predict the trial outcome.
Results:
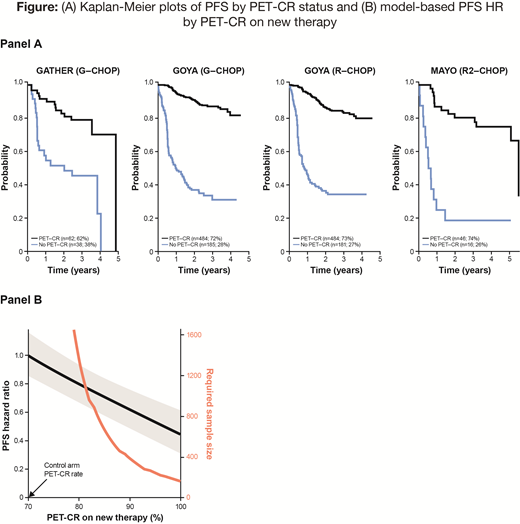
We included 1496 patients (GOYA-R, GOYA-G, GATHER, MAYO, respectively: 665, 669, 100, 62). EOT PET-CR status was determined by the Lugano criteria in GOYA and by IHP in the other two studies. The overall rate of PET-CR was 72%; respectively 72%, 73%, 62% and 74% per arm. Panel A of the Figure shows Kaplan-Meier plots of PFS by PET-CR status. The Bayesian modeled hazard ratio (HR) comparing PET-CR versus nonCR was 0.13 (95% CI: 0.10, 0.15). The model can be used in planning a clinical trial as follows. Suppose a new therapy improves the PET-CR rate from 70% to 85%. Based on our model the expected HR for PFS treatment effect would be 0.71 (95% CI: 0.58, 0.84). The trial sample size required to demonstrate such an improvement with 90% power is about 650 patients, assuming an accrual rate of 50 patients per month and a minimum follow-up time of 12 months. Panel B of the Figure shows the model-based PFS HR and its CI (shaded) as it depends on the PET-CR rate of the new therapy. This plot also shows the estimated total trial sample size (in red), again assuming the relationship between PET-CR and PFS in our model. However, our model may not apply for the new therapy; and the trial could have an adaptive design with final sample size tailored to the accruing information about PET-CR and PFS and their relationship for the therapies in the trial.
Conclusions:
Achieving an EOT PET-CR in newly diagnosed DLBCL is highly predictive of favorable outcome in the populations we considered. The estimated HR is 0.13 for PFS, PET-CR versus nonCR, and it is 0.10 for OS. Based on our model, a treatment that improves PET-CR rate could be reasonably expected to have a benefit on PFS and OS, for the populations we considered. Whether a new therapy, with a different mechanism of action, that improves PET-CR rate will extend PFS in a clinical trial is less clear. An adaptive trial could be initiated based on our model as a hypothesis. This hypothesis can be verified or updated using accruing information in the trial itself. Our model can also help in planning clinical trials for re-estimating sample size during the trial and considering early stopping for futility. Further work should investigate the applicability of PET-CR in predicting PFS and/or OS for treatments that have different mechanisms of action and for other populations of patients.
Acknowledgements: This work was funded by Genentech/Roche.
Berry:Berry Consultants, LLC: Consultancy, Employment, Equity Ownership, Other: Berry Consultants, LLC is a company that provides statistical design and analysis services to pharmaceutical companies (including Genentech/Roche), medical device companies, U.S. NIH cooperative groups, patient advocacy groups, and international consortia. Broglio:Berry Consultants LLC: Employment. Ward:Hoffmann La Roche: Employment, Equity Ownership. Mattiello:F. Hoffmann-La Roche Ltd: Employment. Sahin:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. McGlothlin:Berry Consultants, LLC: Consultancy, Employment. Elliott:Berry Consultants, LLC: Employment. Sehn:Lundbeck: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Janssen-Ortho: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Merck: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Verastem: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Trněný:Abbvie: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Celgene: Consultancy; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria. Vitolo:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; F. Hoffmann-La Roche: Speakers Bureau. Martelli:Servier: Honoraria; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kostakoglu:F. Hoffman-La Roche: Consultancy; Genentech: Consultancy. Nowakowski:F. Hoffmann-La Roche Ltd: Research Funding; Curis: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech, Inc.: Research Funding; MorphoSys: Consultancy, Research Funding; Selvita: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal