Background:
The near ubiquity and persistence of CD20 expression in B-cell malignancies provides a strong rationale for novel CD20-directed therapies.
MT-3724 is a novel engineered toxin body comprised of an anti-CD20 single chain variable fragment genetically fused to Shiga-like toxin A subunit, which forces internalization of CD20-bound MT-3724, irreversibly inactivating ribosomes and triggering apoptosis. As MT-3724 competes with rituximab (RTX) for the same CD20 binding domain, undetectable or low (< 500 ng/mL) RTX levels (RTX-neg) allow potential response to MT-3724. We present final safety and efficacy results from dose escalation to maximum tolerated dose (MTD) in Non-Hodgkin Lymphoma (NHL) subjects (subj) and dose expansion in RTX-neg DLBCL subj.
Methods:
In Part 1, MT-3724 dose was escalated in 21 subj with B-cell NHL according to the 3+3 design based on ≤1 subj exhibiting DLT during Cycle 1 (28 days) in 6 sequential dose cohorts (5, 10, 20, 50, 75, and 100 μg/kg/dose IV over 2 hours 3 times per week for 2 of 4 consecutive weeks) for up to 5 cycles. In Part 2, the safety and efficacy of MT-3724 was further evaluated in 6 RTX-neg subj with DLBCL. Tumor response was assessed by the International Working Group Response Criteria for Clinical Trials (Cheson 2007).
Results:
Twenty-seven subj with NHL (DLBCL:16, composite DLBCL/Follicular Lymphoma (FL): 3, FL: 6, Mantle Cell Lymphoma: 2 were enrolled. Seventeen (63%) were female, mean age was 65 yrs (34-78). Performance status was ECOG 0: 44%, 1: 44%, 2: 11%; median follow-up was 50 (11-372) days; median cycles 2 (1-11).
The most common AEs (%) of all grades were peripheral edema (63), fatigue (41), diarrhea (41), myalgia (41), muscle weakness (33), insomnia (30); 27 subj had AEs (%) with related causality, the most common were: peripheral edema (41), myalgia (33), fatigue (26). The most common related grade (G) ³3 AEs included neutropenia, myalgia, and infections (all 11%); There were 9 related SAEs among 6 subj. One subj died on study from disease progression. MT-3724 was not tolerated at 100 µg/kg/dose (DLTs: G3 pneumonia, G2 ileus). After 2 obese (BMI >35) subj treated at 75 µg/kg/dose in the expansion cohort had G2 capillary leak syndrome (CLS), the MTD was set at 50 µg/kg/dose with a 6000 mg/dose cap. Innate immune responses such as CLS or manifestations thereof (hypotension, hypoalbuminemia, peripheral edema) were noted with increasing frequency with higher doses of MT-3724. No cases of >G2 CLS have been observed.
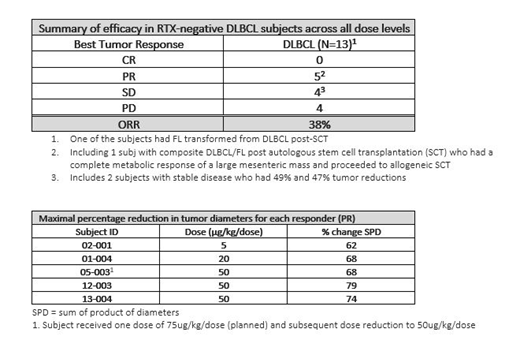
Of the 13 RTX-neg DLBCL subj, 5 (38%) responded across the range of 5 to 50 μg/kg/dose. All responses were partial responses (PRs) including 1 subj with composite DLBCL/FL post autologous stem cell transplantation (SCT) who had a complete metabolic response of a large mesenteric mass and proceeded to allogeneic SCT. Three subj had stable disease (SD) including two with 49% and 47% tumor reductions, one of whom had FL transformed from DLBCL post autologous SCT; another 5 had progressive disease. Given a limited prescribed period of treatment with MT-3724, duration of response cannot be calculated, however, generally the tumor burden in responders decreased with repeat treatment. Among the responders, mean age was 61 (50-76) yrs, and the median number of prior lines of NHL therapy received was 3 (1-8). Subj with detectable baseline RTX levels did not benefit from MT-3724.
Of all 20 subj measured, 6 developed anti-drug antibodies (ADAs) during the first cycle of therapy; 5 developed ADAs at a later timepoint; 6 did not develop ADAs. ADAs were observed in all subj who showed a response, and in 4 of 5 subj with SD. Increasing MT-3724 dose was associated with a dose-dependent peripheral B-cell depletion, evidenced by decreasing CD19+ cells.
Conclusions
MT-3724 is the first CD20 targeted immunotoxin to enter clinical trials. Safety events were mostly mild-moderate, and DLTs were in line with the mechanism of action of MT-3724. A tolerable dose and schedule have been identified: 50 μg/kg/dose up to a maximum of 6000 μg/dose infused over 1 hour on days 1, 3, 5, 8, 10, and 12 of a 21-day cycle. A 38% objective response rate has been observed with monotherapy in a heavily pretreated, RTX-neg DLBCL population; of these subj, 5 were treated at 50 ug/kg (MTD) and 3 of whom responded. The development of ADAs did not preclude benefit of MT-3724. An ongoing phase 2 monotherapy study to confirm efficacy and safety in RTX-neg subj with DLBCL (NCT02361346) is open for recruitment.
Musteata:Institute of Oncology: Employment; Arensia EM: Other: Principal Investigator. Park:BMS: Consultancy, Research Funding; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees; G1 Therapeutics: Consultancy; Teva: Consultancy, Research Funding; Gilead: Speakers Bureau; Seattle Genetics: Research Funding, Speakers Bureau. Burnett:Molecular Templates, Inc.: Employment. Dabovic:Molecular Templates, Inc.: Employment. Williams:Molecular Templates, Inc.: Employment. Higgins:Molecular Templates, Inc.: Employment, Equity Ownership. Persky:Sandoz: Consultancy; Morphosys: Other: Member, Independent Data Monitoring Committee; Debiopharm: Other: Member, Independent Data Monitoring Committee; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal