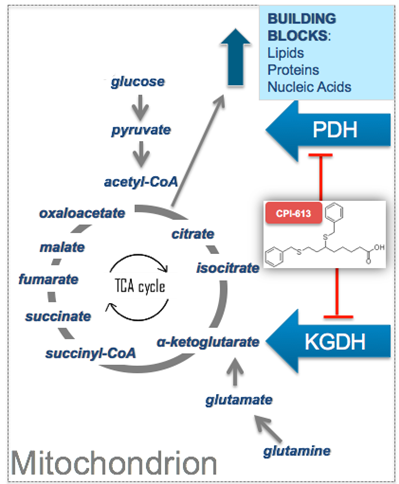
Background: Patients with primary refractory or relapsed Burkitt lymphoma/leukemia (BL) or high-grade B-cell lymphoma with rearrangements of MYC and BCL2 (double hit, DHL) and/or BCL6 (triple hit, THL) have a dismal prognosis with patients rarely achieving meaningful remissions following second line therapy. No standard therapeutic approach exists for this group. The characteristic hallmark of these diseases is a dysregulated MYC oncogene with downstream effects on both proliferation and highly glycolytic metabolism which use tricarboxylic acid (TCA) cycle intermediates as biosynthetic precursors. CPI 613® (devimistat) is a non-redox active analogue of lipoic acid, a required cofactor for two key mitochondrial enzymes of the TCA cycle: pyruvate dehydrogenase and alpha ketoglutarate dehydrogenase. Disruption of these enzyme activities results in a shutdown of ATP and biosynthetic-intermediate production leading to cancer cell death by apoptosis or necrosis. In the initialphase I trial a patient with multiply refractory BL had a partial remission on CPI 613 sustained for over one year prior to surgical resection. Given the rarity of these types of responses in multiply relapsed BL we initiated a phase II trial to further explore efficacy. CPI-613® has FDA orphan status for BL.
Study Design and Methods: NCT03793140 is a multicenter study enrolling 17 patients on each of two cohorts BL or DHL/THL. Patients must have had one prior therapy or are refusing standard of care, measurable disease or isolated bone marrow involvement, and must not be within 3 months of a prior stem cell transplant. Patients with active central nervous system (CNS) parenchymal disease are excluded, but those with leptomeningeal disease are eligible if the CSF is negative for lymphoma for more than 4 weeks and the maintenance intrathecal/intraOmmaya therapy is ongoing . CPI 613 is given by central line over 2 hours daily x 5 days for two 14-day cycles and then in 21 day cycles. With a primary objective of overall response, treatment can be used as a bridge to transplant. Secondary endpoints include duration of response, progression-free survival (PFS) and overall survival (OS). Primary and secondary outcomes will be correlated with pre-treatment biomarkers including variances in serum metabolites, immunohistochemistry staining for PDH, KGDH, PDKs1-4, SOD2 and pretreatment cytokine profiles. Biostatistics include a Simon minimax two-stage design for efficacy after the first 10 patients in each of the BL and DHL/THL cohorts separately analyzed. Additionally, an interim analysis for toxicity will be conducted after the first 10 study participants have completed two complete cycles or have come off study.
Noy:Raphael Pharma: Research Funding; Pharamcyclics: Research Funding; Janssen: Consultancy; Medscape: Honoraria; Prime Oncology: Honoraria; NIH: Research Funding. Pardee:Rafael Pharmaceuticals: Consultancy, Research Funding; Karyopharm: Research Funding; Pharmacyclics/Janssen: Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; CBM Bipharma: Membership on an entity's Board of Directors or advisory committees; Spherix Intellectual Property: Research Funding. Abramson:AbbVie Inc, Amgen Inc, Bayer HealthCare Pharmaceuticals, Celgene Corporation, EMD Serono Inc, Genentech, Gilead Sciences Inc, Janssen Biotech Inc, Juno Therapeutics, a Celgene Company, Karyopharm Therapeutics, Kite Pharma Inc, Merck, Novartis, Seattle Gen: Consultancy. Dunleavy:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Luther:Raphael: Employment.
CPI 613 devimistat is a is a non-redox active analogue of lipoic acid, a required cofactor for two key mitochondrial enzymes of the TCA cycle
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal