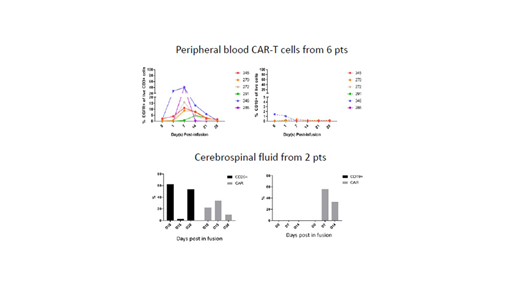
Background: Prognosis is generally poor for patients (pts) with primary or secondary central nervous system (CNS) lymphoma. We report data from such patients treated on the ongoing Phase 1 trial investigating an autologous CD19 specific, hinge-optimized, CD28 costimulatory chimeric antigen receptor with a truncated eGFR for the treatment of B-cell non-Hodgkin lymphomas (NHL) at City of Hope National Medical Center. Methods: Eligible pts had confirmed B-cell NHL with relapsed/refractory (r/r) disease and patients with CNS lymphoma (history of or active at the time of enrollment) could enroll. After lymphodepleting chemotherapy, CD19-targeting CAR-T cells were administered at 1 of 2 dose levels (DL): DL1 = 200 million (M) cells and DL2 = 600M cells. All patients received levetiracetam for seizure prophylaxis. Results: At the time of data lock (06/2019), three (3) patients with primary CNS lymphoma and four (4) with secondary CNS lymphoma had received CAR-T cells. Five (5) pts were treated at DL1 and two (2) were treated at DL2.The median (range) age was 53.0 (47.0-70.8) years and median (range) number of prior lines of systemic therapy was 6 (4-12). No pts had grade (G) 3 or higher cytokine release syndrome (CRS) or neurological toxicities (NT). Two (2) pts received corticosteroids and three (3) pts received tocilizumab for CAR-T cell associated grade 1-2 NT and CRS respectively. Other toxicities were predominantly cytopenias related to the lymphodepleting chemotherapy. There were no treatment-related deaths. 4 pts had an objective response: 1 complete remission and 3 partial remissions. Representative peripheral blood and cerebrospinal fluid samples are shown in the Figure. Conclusions: In this ongoing City of Hope CAR-T cell trial targeting CD19 in patients with r/r B-cell NHL, promising results were seen in patients with primary and secondary CNS lymphoma, a population of pts with a high unmet medical need. No grade 3 or higher CRS or NT were noted. Expansion phase enrollment continues currently and an intraventricular route of CAR-T cell delivery will also be evaluated for potentially improved antitumor effects. Clinical trial information: NCT02153580.
Siddiqi:Janssen: Speakers Bureau; Seattle Genetics: Speakers Bureau; BeiGene: Research Funding; Celgene: Research Funding; TG Therapeutics: Research Funding; Kite: Research Funding; Astra Zeneca: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Juno: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding, Speakers Bureau. Palmer:Gilead Sciences: Consultancy. Popplewell:City of Hope: Employment. Herrera:Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; AstraZeneca: Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; Pharmacyclics: Research Funding; Immune Design: Research Funding; Kite Pharma: Consultancy, Research Funding. Budde:F. Hoffmann-La Roche Ltd: Consultancy.
City of Hope CAR-T cells are not FDA approved.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal