Introduction
Since primary mediastinal B-cell lymphoma (PMBCL) is a rare cancer, a prospective clinical trial is challenging and optimal therapy for relapsed/refractory PMBCL (rrPMBCL) has not been defined. The current clinical standard-of-care has been extrapolated from retrospective series or from subgroup analyses of prospective trials designed for diffuse large B-cell lymphoma. Novel salvage strategies are therefore urgently required in rrPMBCL.
Methods
This single-arm, open-label, phase II trial enrolled patients with heavily pre-treated, rrPMLBCL who have bulky disease (the longest diameter ≥5 cm) and have relapsed or refractory disease after ≥ 2 lines of prior therapy. All enrolled patients received combining PD-1 blockade using Camrelizumab with GVD chemotherapy (gemcitabine, vinorelbine and pegylated liposomal doxorubicin)every 3 weeks until the patients achieved second assessable complete response or up to 12 cycles, followed by maintenance camrelizumab monotherapy up to 1 year. Safety was assessed by CTCAE v5.0 in the population consisted of all patients who received ≥ 1 dose of study drug. Clinical response was evaluated by computed tomography (CT) every 2 cycles and by positron emission tomography-computed tomography (PET-CT) every 4 cycles according to the Lugano Response Criteria for Non-Hodgkin's Lymphoma. Peripheral blood samples were collected before every cycle and tumors were biopsied prior to initiation of therapy for relevant biomarker analysis. The primary end points were objective response rate (ORR) and safety/tolerability. Key secondary end points were complete response rate (CRR), progression-free survival (PFS) and overall survival (OS).
Results
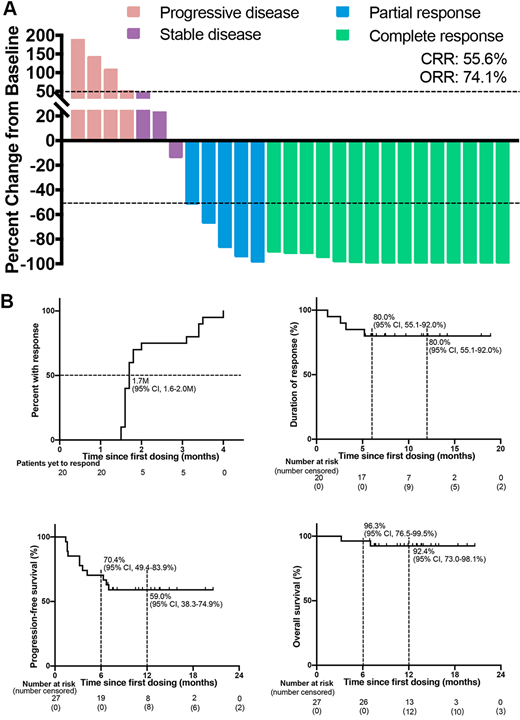
At the analysis cutoff date (March 31, 2019), 27 rrPMLBCL patients were enrolled: median age 30 years (range: 18-45), 52% female (14/27), median 3 lines of prior therapy (range: 2-6), median 6 cycles of rituximab-containing regimens (range: 2-10). In all 27 response-evaluable patients with heavily pretreated rrPMBCL, Camrelizumab plus GVD achieved unprecedented OR (74.1% [95% CI 55.3-86.8]) and CR rates (55.6% [95% CI 37.3-72.4]), with rapid and lasting responses. The median time to response of 20 patients achieving response was 1.7 months (95% CI 1.6-1.8), while 21 (78.0% [95% CI 59.2-89.4]) patients had a decrease in target lesions at the first tumor scan assessment. With a median follow-up of 12.5 months (range, 3.2-20.6), the median duration of response (DOR) and progression-free survival (PFS) were not reached. Censored at 1-year, the DOR and PFS was 80.0% (95% CI 55.1-92·0) and 59·0% (95% CI 38·3-74·9), respectively. Sixteen of 20 (80%) responders, including all 15 patients achieving complete response, had an ongoing response at data cutoff. The addition of Camrelizumab to the GVD regimen did not appear to exacerbate treatment-related adverse events. Any-grade and grade 3 treatment-related adverse events was occurred in 25 (93%) and 9 (33%) patients, respectively. No grade 4 or 5 adverse events were reported. The most common treatment-related adverse events were neutropenia (70%), leukocytopenia (52%), pruritus (30%) and anaemia (26%). An exploratory analysis undertaken to identify potential biomarkers of response indicated a role for serum levels of IFN-γ, IL-10 and sFas to predict the clinical outcome.
Conclusions
Patients with rrPMBCL represent a particularly challenging population to treat, with few effective treatment options in the context of a dismal prognosis. Aside from palliation, new salvage therapeutic strategies are urgently needed. Camrelizumab plus GVD chemotherapy should be considered as a compelling new standard of salvage therapy with promising efficacy and manageable safety profile for patients with rrPMBCL.
Rasko:Cure The Future Foundation: Membership on an entity's Board of Directors or advisory committees; Genea: Equity Ownership; Takeda: Honoraria; Rarecyte: Consultancy, Equity Ownership; Gene Technology Technical Advisory, Australian Government: Other: Advisory committee; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal