Background: While Hodgkin lymphoma (HL) is highly curable with standard chemotherapy in high-resource settings, there are few reports of HL treatment in low-resource settings. In Rwanda, a treatment protocol using six cycles of ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, dacarbazine) without radiotherapy has been implemented at two rural district hospitals. Here we report on the feasibility of this approach, our patient characteristics, and outcomes.
Methods: We conducted a retrospective cohort study of all patients with biopsy-confirmed HL seen at Butaro and Rwinkwavu hospitals between October 2009 and June 2018. Data were extracted from clinical charts and analyzed using descriptive statistics and Kaplan-Meier logrank testing.
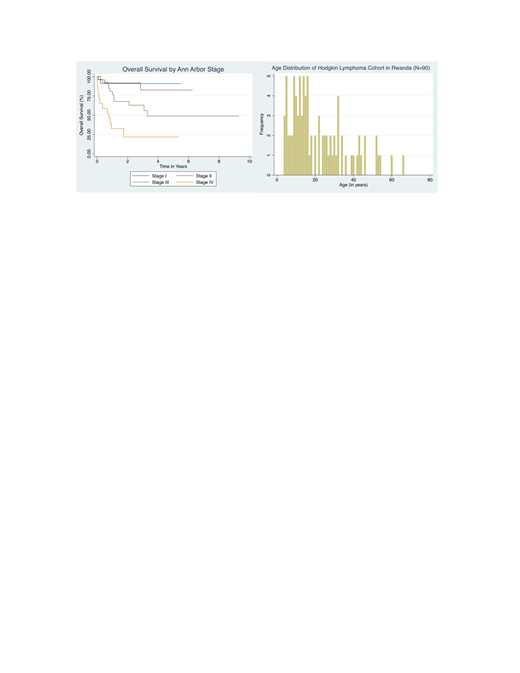
Results: Ninety HL patients were seen at Butaro (n=85) and Rwinkwavu (n=5); 58% male, median age 16 (IQR 10.6-30.5) with 54% under age 18. Eleven (12%) were HIV positive. Mean duration of presenting symptoms at the time of intake was 54 weeks; 70% had B symptoms. Nodular sclerosis was the predominant histological subtype (47%) followed by mixed cellularity (28%). Of 24 biopsy specimens evaluated for EBV, 14 (58%) were positive, 10 (42%) negative. Most patients were staged with chest x-ray (81%); fewer had abdominal ultrasound (34%), CT (21%), or bone marrow biopsy (20%). Resulting Ann Arbor stages were I (17%), II (28%), III (32%), IV (20%), and undetermined (3%). Median time from initial biopsy to first dose of ABVD was 6.1 weeks (IQR 3.6-11.9). Of 76 patients who started ABVD, 56 (74%) completed all 6 cycles; the leading reasons for discontinuation were loss to follow up (n=9) and death (n=7). Median time to completion of the 24-week ABVD regimen was 26.1 weeks; 51 patients (67%) experienced at least one treatment delay. Neutropenia, social factors, and infection were the most common reasons for delays. Dose reductions were rare. Mean dose intensity over 6 cycles was 87.2% calculated per Owadally, et al. 2010; <86% in 40% of patients, 86-97% in 32%, and >97% in 28%. Of the 76 patients started on ABVD, 34 (45%) are in clinical remission, 21 (28%) are deceased, 2 (3%) referred to palliative care, and 19 (25%) are lost to follow up at a median interval of 48 months from intake. Univariate analysis demonstrates that stage III-IV (p=0.0000), ECOG performance status 2-4 (p=0.0004), hemoglobin <10.5 g/dL (p=0.01), WBC > 15,000 mm3 (p=0.05), B symptoms (p=0.004), and extranodal disease (0.0004) were associated with worse survival.
Conclusions: We observed a strikingly younger age distribution in our cohort compared to the classic bimodal distribution reported in high income countries, suggesting biologic differences that warrant further investigation. Treating HL with standard chemotherapy in a low-resource setting through international partnership is feasible, and nearly half of patients who complete treatment may experience a clinically significant remission with this approach. Late presentation, treatment delays, and loss to follow up are among major reasons to explain the discrepancy in survival compared to high income countries. Further efforts should tackle these identified barriers to achieve better survival outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal