Introduction: Peripheral T cell lymphoma (PTCL) is a very heterogenous disease and corresponds to approximately 15% of all non-Hodgkin lymphoma cases. PTCL is divided into several subtypes, however, PTCL not otherwise specified (PTCL-NOS) is the most frequent, with a proportion of 26% of all PTCL cases. There is a lack of demographic and clinical data about PTCL-NOS in middle- and low-income countries, where patients' access to early diagnosis and otherwise standard care might be suboptimal. The objective of this study is to describe the population of PTCL-NOS patients in Latin America, specifically from the countries conforming the "Grupo Latinoamericano de Linfomas" (GELL), in order to better understand clinical behavior and find possible prognostic factors that might prognosticate overall survival (OS). We specifically evaluated the neutrophil/lymphocyte ratio (NLR) and serum albumin as potential prognostic factors.
Methods: An observational, retrospective and analytical study was conducted during the period from January 2000 through January 2018. A total of 200 Latin American patients with a pathological diagnosis of PTCL-NOS were included. Clinical data were gathered from clinical records. NLR ≥4 and serum albumin ≤3.5 g/dl were considered adverse prognostic factors. Median Overall Survival (mOS) and 5-year Overall Survival (5y-OS) rates were estimated using the Kaplan-Meier method. Univariate and multivariate Cox proportional-hazard regression analyses were performed to identify adverse prognostic factors for OS. Data were analyzed and interpreted using STATA 15.
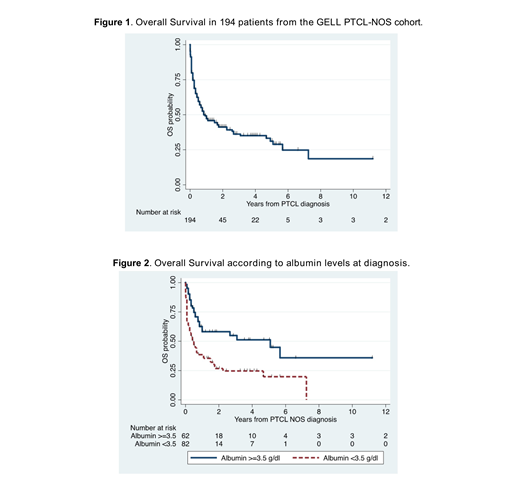
Results: A total of 200 patients with a diagnosis of PTCL-NOS were included. 50% of patients were ≥60 years, 57% were male, 50% had ECOG ≥2, 40% had elevated serum Lactate Dehydrogenase (LDH) level, 70% showed stage III/IV disease, bone marrow involvement was present in 37% of patients, B symptoms in 65%, 33% presented with hemoglobin levels <10 g/dL. The International Prognostic Index (IPI) score was high-intermediate in 33% and high in 14% of cases. The Prognostic Index for PTCL-U (PIT) risk score was high-intermediate in 32% and high in 26% of cases. Serum albumin <3.5 mg/dl was seen in 58%, and NLR ≥4 in 37% of patients. Median OS (mOS) for the entire cohort was 0.83 years (95% CI 0.58-1.75) and 5-year OS rate was 31% (95% CI 23-40%). Patients with serum albumin levels <3.5 g/dL had mOS of 0.42 years (95% CI 0.25-0.75) and 5-year OS rate of 20% (95% CI 9-33%), while patients with albumin ≥3.5 g/dL had mOS of 5.1 years (95% CI 0.83-not reached) and 5-year OS rate of 51% (95% CI 36-65%) (log-rank p<0.001). The mOS for NLR <4 was 1.67 years (95% CI 0.75-4.92) with 5-year OS rate of 37% (95% CI 25-48%) while for NLR ≥4, the mOS was 0.58 years (95% CI 0.25-1.00) and 5-year OS rate was 23% (95% CI 12-36%) (log-rank p=0.02). Cox proportional Hazard regression multivariate analyses found serum albumin <3.5 g/dL (HR 1.83, 95% CI 1.10-3.05; p=0.02) and ECOG ≥2 (HR 1.95, 95% CI 1.15-3.30; p=0,01) were associated with a worse OS. Serum albumin remained an adverse prognostic factor for OS after adjustment for the IPI and the PIT scores (HR 1.66, 95% CI 1.01-2.75; p=0.047, and HR 1.70, 95% CI 1.03-2.80; p=0.038, respectively).
Conclusion: This multi-institutional Latin American study showed that serum albumin level <3.5 g/dL was an adverse prognostic factor for OS, independent from the IPI and the PIT scores, in Latin American patients with a diagnosis of PTCL-NOS. The survival rates of Latin American patients with PTCL-NOS appear lower than in developed countries.
M:Merck-Sharp-Dome: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Roche-Mexico: Consultancy, Speakers Bureau. Peña:Novartis: Other: Congress inscription and flights; Tecnofarma: Other: Congress inscription and flights; Roche: Other: Congress inscription and flights; Biotoscana: Other: Congress inscription and flights; Janssen: Other: Congress inscription and flights; Pfizer: Membership on an entity's Board of Directors or advisory committees. Paredes:Tecnofarma: Honoraria. Rojas:ROCHE: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees; ABBVIE: Membership on an entity's Board of Directors or advisory committees. Abello:Takeda: Other: Participation in advisory board meeting. Castillo:Abbvie: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal