Background: Adult T-cell leukemia/lymphoma (ATLL) is an aggressive, peripheral T-cell neoplasm associated with the human T-cell leukemia virus type 1 (HTLV-1). HTLV-1 infects up to 10 million people worldwide and is most endemic in Southwestern Japan, the Caribbean basin, South America and Western Africa. In Latin America, highest prevalence is found in Haiti, Jamaica, Dominican Republic, Brazil and Peru. ATLL has a poor prognosis, with shorter overall survival (OS) relative to other peripheral T-cell lymphomas. Although current prognostic models require extensive radiologic and laboratory investigations, oftentimes they are not readily available in most Latin American countries, hence, a simple prognostic model is useful. We aim to identify and then validate a simple clinical prognostic model for ATLL in the Latin American population by analyzing clinical parameters and only laboratory tests that are widely available across Latin American countries.
Patients and Methods: We retrospectively analyzed patients (pts) diagnosed with ATLL between January 1987 and December 2018. Aggressive ATLL cases were classified according to the Shimoyama criteria into acute (A) and lymphomatous (L). Cox regression modeling was performed on several clinical and laboratory parameters in two independent cohorts: first, a learning cohort (LC) of ATLL pts diagnosed and managed at two tertiary hospitals in Chile and Peru, and then a cohort of ATLL pts from a tertiary hospital in Miami was used to validate the model (validation cohort, VC). OS curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional-hazard regression models were fitted.
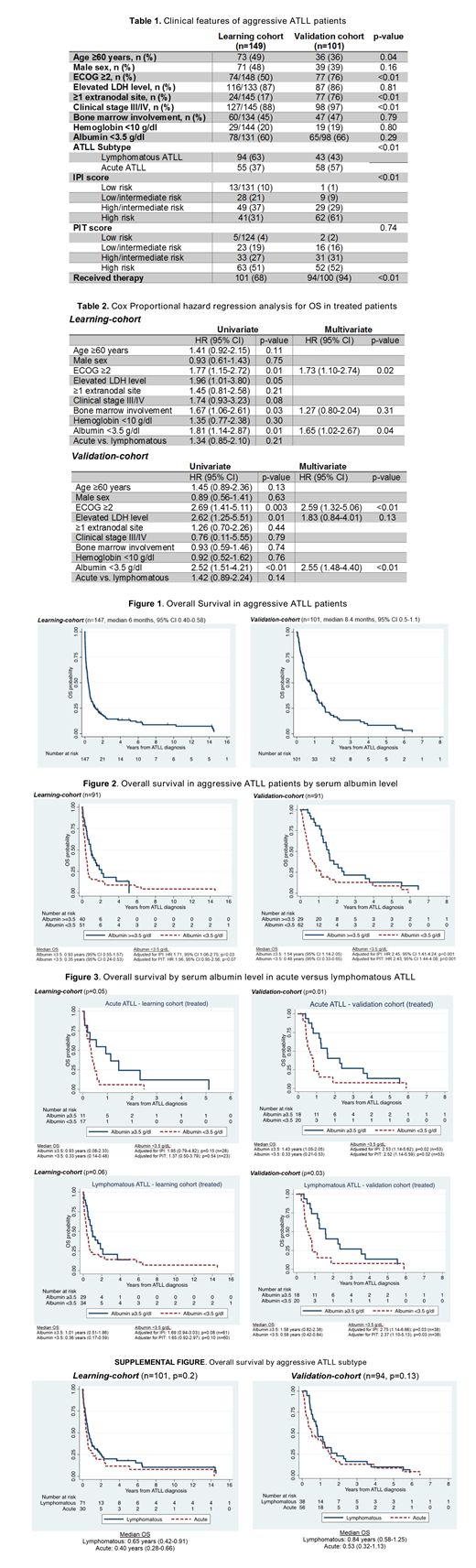
Results: A total of 149 pts (A=55, L=94) in the LC, and 101 pts (A=58, L=43) in the VC were identified, with 101 and 94 pts receiving therapy in each cohort, respectively. Clinical features are shown in Table 1. In both cohorts, there was a young (<60 years, LC=51%, VC=64%), and female predominance (LC=52%, VC=61%). Pts in the LC had a better performance status compared to the VC (ECOG ≥2, LC=50% vs. VC=76%). Pts in the VC had advanced stages of disease (stage III/IV, LC=88% vs. VC=97%), and ≥1 extranodal involvement (LC=17% vs. VC=76%) compared to the LC. High LDH, low serum albumin (<3.5 g/dL), bone marrow involvement and anemia (hemoglobin <10 g/dL) were no different in both cohorts. The calculated International Prognostic Index (IPI) and Prognostic Index for T-cell lymphoma (PTI) scores are presented in Table 1. Most pts in the VC had high risk IPI and PIT scores (High risk: IPI LC=21% vs. VC=62%; PIT LC=38% vs. VC=51%) compared to the LC that had more low and low/intermediate IPI and PIT scores (Low risk: IPI LC=13% vs. VC=0%; PIT LC=5% vs. VC=0%; Low/intermediate risk: IPI LC=26% vs. VC=7%; PIT LC=23% vs. VC=16%). The median OS was 6 and 8.4 months in the LC and VC, respectively (Figure 1).
In the univariate and multivariate analysis, ECOG ≥2 and serum albumin <3.5 g/dL were both associated with a worse OS (Table 2). When adjusted to IPI and PIT scores, serum albumin <3.5 g/dL was a negative prognostic factor, independent of IPI score, and a trend in PIT score, in the LC (adjusted for IPI: HR 1.71, 95% CI 1.06-2.75; p=0.03 / PIT: HR 1.56, 95% CI 0.95-2.56; p=0.07), but independent prognostic factor from both, IPI and PIT scores, in the VC (adjusted for IPI: HR 2.45, 95% CI 1.41-4.24; p=0.001 / PIT: HR 2.43, 95% CI 1.44-4.08; p=0.001) (Figure 2). Comparable results were found when investigating by ATLL subtype, with results trending towards significance for OS, IPI and PIT scores in the LC, but then validated in the VC (Figure 3).
Conclusions: To the best of our knowledge, this is the largest retrospective study evaluating the clinical features of HTLV-1 related ATLL and impact on disease outcome in Latin America. We have validated a simple prognostic model in pts with aggressive ATLL. Our results suggest that a serum albumin level of less than 3.5 g/dL is a reliable, and independent prognostic factor for survival in aggressive ATLL. This prognostic model could be used to complement or modify existing and widely used international prognostic indexes for lymphoma. This simple paradigm could be useful in validating treatment outcomes after chemotherapy or highly needed new approaches for ATLL in prospective studies, particularly in developing countries where the absence of sophisticated laboratory and imaging tests hinder treatment decisions.
Peña:Novartis: Other: Congress inscription and flights; Tecnofarma: Other: Congress inscription and flights; Roche: Other: Congress inscription and flights; Biotoscana: Other: Congress inscription and flights; Janssen: Other: Congress inscription and flights; Pfizer: Membership on an entity's Board of Directors or advisory committees. Rojas:Pfizer: Membership on an entity's Board of Directors or advisory committees; ABBVIE: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees; ROCHE: Membership on an entity's Board of Directors or advisory committees. Paredes:Tecnofarma: Honoraria. Abello:Takeda: Other: Participation in advisory board meeting. M:Merck-Sharp-Dome: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Roche-Mexico: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal