Background:
Interleukin-2-Inducible T-Cell Kinase (ITK) is a Tec-family, non-receptor tyrosine kinase expressed in T-cells that plays a key role in T-cell receptor (TCR) signaling, which is required for development and differentiation of T-cells. In T-cell lymphoproliferative disorders, expression of the TCR and its downstream signaling components are maintained, which suggests malignant cells may exploit this growth and survival pathway to their advantage. Professional antigen-presenting cells abundant in the lymphoma microenvironment may provide antigen to drive TCR signaling through ITK, which is expressed in a variety of T-cell lymphomas. CPI-818 is a first-in-class, irreversible ITK inhibitor with a high degree of selectivity for ITK. By inhibiting ITK, CPI-818 blocks signaling pathways and is efficacious in murine models of T-cell lymphomas. Furthermore, the safety and efficacy of CPI-818 in companion dogs with spontaneously-occurring T-cell lymphomas have been evaluated. Following oral, BID dosing of CPI-818 to dogs, evidence of anti-tumor activity including complete and partial responses was observed. CPI-818 was well tolerated in dogs with no change in normal lymphocyte counts. Thus, the preclinical evidence supports the evaluation of CPI-818 in clinical trials in patients with T-cell malignancies. To test this clinically, a phase 1/1b dose-escalation and dose-expansion trial of CPI-818 has been initiated in patients with relapsed/refractory T-cell lymphoma.
Methods:
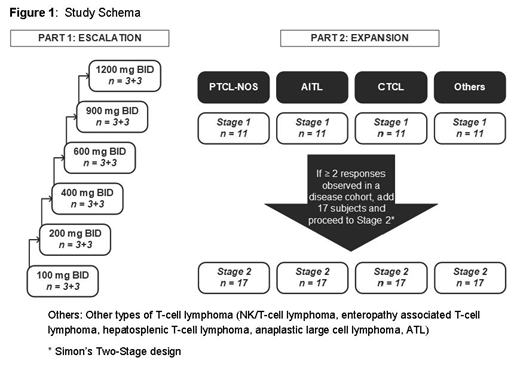
The trial will enroll patients with various types of T-cell lymphoma (PTCL and CTCL) who have progressed on, refractory to, relapsed, or intolerant to at least 2 standard therapies; age ≥ 18 years; have ECOG status 0-1; adequate organ function; and without any other condition that would contraindicate the use of the investigational product. A dose Escalation part with 3 + 3 (+ optional 3) design will consist of up to 6 ascending dose levels (100, 200, 400, 600, 900, and 1200 mg) of CPI-818. In the Dose Expansion part, there will be 4 disease-specific expansion cohorts (AITL, PTCL-NOS, CTCL and other T-cell lymphoma), and each cohort may enroll up to a maximum of 28 subjects/cohort based on a two-stage expansion design- Study Details in Figure 1. Patients will receive CPI-818 orally BID at the assigned dose level continuously up to sixteen 21-day cycles, until progression or unacceptable toxicity.
The primary objectives of the study are to evaluate the safety and tolerability of CPI-818 in ascending dose levels and to establish the maximum tolerated dose or the maximum administered dose of CPI-818. Secondary objectives include evaluating pharmacokinetics/ pharmacodynamics, assessing the anti-tumor activity of CPI-818 and identifying potential biomarker signals. Adverse events and dose-limiting toxicities of CPI-818 will be assessed. The PK profile of CPI-818 will be characterized by PK parameters in plasma. Efficacy will be assessed by the investigator using standard response criteria: Lugano Classification for PTCL patients, Consensus Statement for Response for CTCL patients, and the international consensus modification of Japan Clinical Oncology Group criteria for adult T-cell lymphoma patients. ITK occupancy/engagement in peripheral blood T cells and potential predictive biomarkers associated with anti-tumor activity in tumor tissue and blood samples will be evaluated.
Study Status:
This study is currently enrolling. US NIH Clinical Trials Registration Number NCT03952078.
Mobasher:Corvus Pharmaceuticals: Employment, Equity Ownership. Miller:Corvus Pharmaceuticals: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Janc:Corvus Pharmaceuticals: Employment, Equity Ownership. Kwei:Corvus Pharmaceuticals: Employment, Equity Ownership. Buggy:Corvus Pharmaceuticals: Employment, Equity Ownership. Luciano:Corvus Pharmaceuticals: Employment, Equity Ownership. Mohammady:Corvus Pharmaceuticals: Employment, Equity Ownership. Kim:F. Hoffmann-La Roche Ltd: Research Funding; Celltrion: Research Funding; Novartis: Research Funding; Donga: Research Funding; Kyowa-Kirin: Research Funding; Novartis: Research Funding; J + J: Research Funding. Kim:Portola Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Medivir: Honoraria, Membership on an entity's Board of Directors or advisory committees; Neumedicine: Research Funding; Elorac: Research Funding; Corvus: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Hakko Kirin: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty Seven Inc: Research Funding; Horizon: Research Funding; Galderma: Research Funding; Merck: Research Funding; Trillium: Research Funding; miRagen: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Soligenix: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Khodadoust:Corvus Pharmaceuticals: Research Funding. Horwitz:Portola: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Affimed: Consultancy; Seattle Genetics: Consultancy, Research Funding; Astex: Consultancy; Kyowa Hakko Kirin: Consultancy; ADCT Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Affimed: Consultancy; ADCT Therapeutics: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Affimed: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forty-Seven: Research Funding; ADCT Therapeutics: Research Funding; Trillium: Research Funding; Mundipharma: Consultancy; Kyowa Hakko Kirin: Consultancy; Miragen: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Mundipharma: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Forty-Seven: Research Funding; Aileron: Research Funding; Miragen: Consultancy; Mundipharma: Consultancy; Miragen: Consultancy; Seattle Genetics: Consultancy, Research Funding; Forty-Seven: Research Funding; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Aileron: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Affimed: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy; Astex: Consultancy; Trillium: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Trillium: Research Funding; Forty-Seven: Research Funding; Aileron: Research Funding; Seattle Genetics: Consultancy, Research Funding; Kura: Consultancy; Kyowa Hakko Kirin: Consultancy; Portola: Consultancy; Astex: Consultancy; Innate Pharma: Consultancy; Celgene: Consultancy, Research Funding; Kura: Consultancy; Innate Pharma: Consultancy; Innate Pharma: Consultancy; Kura: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Kura: Consultancy; Innate Pharma: Consultancy; Trillium: Research Funding; Miragen: Consultancy; Mundipharma: Consultancy; Portola: Consultancy. Radeski:Corvus Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal