Background: Treatment practices for patients with limited-stage DLBCL are varied and include combined-modality treatment (3 cycles immunochemotherapy + radiation therapy) or immunochemotherapy alone. Since 2005, patients (pts) in BC with limited-stage DLBCL (stage I/II, no B-symptoms, mass < 10cm) have been treated according to a PET-guided algorithm. Following 3 cycles of R-CHOP, pts undergo FDG-PET/CT scan; PET-negative pts receive one additional cycle of R-CHOP, while PET-positive pts receive involved-site radiation therapy (RT). We present long-term follow-up of this population-based experience.
Methods: Using the BC Cancer Lymphoid Cancer Database we identified all pts who were diagnosed with limited-stage DLBCL from Mar 2005 to February 2019 and underwent a PET/CT after 3 cycles of curative-intent R-CHOP, in keeping with the PET-guided policy. Pts with primary CNS, primary testicular, PMBCL, PTLD, and transformed/discordant/composite lymphoma were excluded. Pts with evidence of progressive disease prior to or on mid-treatment PET/CT were also excluded. All PET/CT scans were performed and reviewed centrally. Prior to 2014, interpretation was based on the International Harmonization Project (IHP) criteria, and subsequently according to Deauville criteria. Importantly, uptake greater than the mediastinal blood pool was consistently used to determine PET-positivity (ie. PET-positive by IHP, or D3-5 by Deauville).
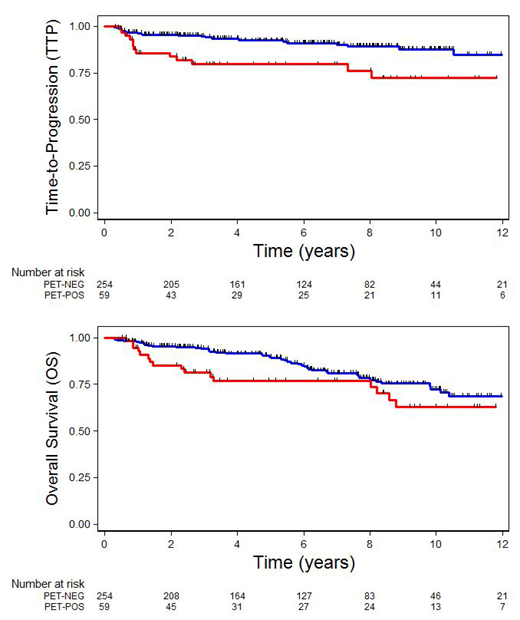
Results: Clinical characteristics of the 319 pts identified are as follows: median age, 68 y (range 19-92); 48%, male; 59%, stage I; 41%, stage II; 8%, PS>1; 13%, elevated LDH; 52%, at least 1 extranodal site; 37%, mass size ≥ 5cm. Stage-modified IPI risk score: 19%, 0; 45%, 1; 27%, 2; 9%, 3-4. Median follow-up is 6.25 yrs (range 0.42-14.25). After 3 cycles of R-CHOP: 254 pts (80%) were PET-negative; 59 pts (18%) were PET-positive; and 6 pts (2%) were considered PET-indeterminate (by IHP). Elevated serum LDH (p<0.001), mass size ≥ 5cm (p=0.008) and stage-adjusted IPI (p=0.025) were predictive of PET-positive status. Of the 254 PET-negative pts, 234 (92%) completed treatment with one additional cycle of R-CHOP, 7 (3%) stopped treatment after 3 cycles R-CHOP and 13 (5%) received RT due to poor chemotherapy tolerance or physician choice. 21/254 PET-negative pts have relapsed and 2/254 died of treatment-related toxicity. 8/21 (38%) PET-negative relapses were late, occurring more than 4 years post initial diagnosis. 55/59 (93%) PET-positive pts received RT, 2 pts refused RT and received only 3 cycles R-CHOP, and 2 pts received 1 additional cycle of R-CHOP alone due to physician preference. 13/59 PET-positive pts have relapsed, only 2/13 (15%) relapses were late >4 years from diagnosis. Of the 6 PET-indeterminate patients, 2 received RT and 3/6 have relapsed. Overall 5-year time-to-progression (TTP) censoring deaths from unrelated causes is 89% (92% for PET-negative and 80% for PET-positive pts). Overall 5-year PFS is 84% (88% for PET-negative and 74% for PET-positive pts) and 5-year OS is 87% (90% for PET-negative and 77% for PET-positive pts). On univariate analysis, age>60, PS>1, stage-modified IPI, and PET status were significant predictors of TTP. On multivariate analysis controlling for age>60, stage, PS, LDH, presence of extranodal involvement, mass size ≥ 5cm, and PET status, only age (p=0.002) and PET status (p=0.004) remained independent predictors of TTP.
Conclusion: Using a PET-guided approach to treatment, the majority of patients with limited-stage DLBCL have negative PET scans after 3 cycles of R-CHOP. Patients with negative PET scans have an excellent outcome when treated with 4 cycles of R-CHOP alone, without exposure to radiation. Patients with a positive PET scan who complete treatment with radiation therapy have a slightly less favorable outcome, and may be appropriate for alternative approaches. A detailed analysis of patterns of relapse, as well as efforts to identify clinical factors, PET parameters and biomarkers associated with poor outcome and delayed relapse are ongoing.
Sehn:Acerta: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Janssen-Ortho: Honoraria; Merck: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Acerta: Consultancy, Honoraria. Scott:NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoSting [Institution], Research Funding; Roche/Genentech: Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy. Gerrie:Lundbeck, Seattle Genetics: Consultancy, Honoraria. Freeman:Seattle Genetics, Janssen, Amgen, Celgene, Abbvie: Consultancy, Honoraria. Pickles:TarSera: Honoraria, Other: Participated in advisory board meeting; Abbvie, Sanofi: Consultancy, Honoraria; Astellas Inc.: Research Funding. Savage:Seattle Genetics, Inc.: Consultancy, Honoraria, Research Funding; BMS, Merck, Novartis, Verastem, Abbvie, Servier, and Seattle Genetics: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal