Introduction: Copanlisib, a pan-class I phosphatidylinositol 3-kinase inhibitor, is approved in the US for the treatment of patients (pts) with relapsed follicular lymphoma (FL) who have received ≥2 prior systemic therapies; it also has Breakthrough Therapy designation for pts with relapsed marginal zone lymphoma who have received ≥2 prior therapies. The Phase II CHRONOS-1 study in pts with indolent lymphoma treated with copanlisib (NCT01660451; Part B) demonstrated an objective response rate of 59.2% with manageable safety and low rates of severe adverse events (AEs) (Dreyling M et al. J Clin Oncol 2017). We report pooled safety data from 8 Phase I and II studies of pts with hematologic malignancies following long-term treatment with copanlisib.
Methods: Pts with hematologic malignancies treated with copanlisib monotherapy in completed open-label Phase I or II studies were included. Copanlisib 60 mg (i.v.) was administered intermittently on days (d) 1, 8, and 15 of a 28-d cycle until disease progression (PD) or unacceptable toxicity. AEs were reported using MedDRA (v.21.1) and assessed by time of onset (worst grade or new AEs occurring ≤180 d, 181-360 d, or ≥361 d) and treatment duration.
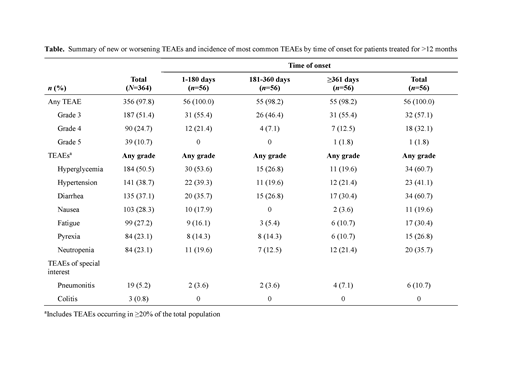
Results: A total of 364 pts received copanlisib (data cut-off Feb 2019). Median age was 65 years (range 22-93); the predominant histology was FL (42.0%). At data cut-off, 34 pts (9.3%) remained on treatment. Duration of treatment ranged from 0.2 to 62.1 months, with 56 pts (15.4%) treated for >1 year. All-grade treatment-emergent AEs (TEAEs) were reported in 97.8% of pts; 51.4% had TEAEs of worst grade [g] 3, and 24.7% had TEAEs of worst g4. The most common (≥20%) TEAEs (all grade / g3 / g4) were hyperglycemia (50.5% / 28.3% / 4.4%), hypertension (38.7% / 29.1% / 0%), diarrhea (37.1% / 4.9% / 0%), nausea (28.3% / 1.4% / 0%), fatigue (27.2% / 2.7% / 0%), pyrexia (23.1% / 3.0% / 0%), and neutropenia (23.1% / 9.3% / 9.6%). Hyperglycemia and hypertension were infusion related, transient, and manageable. Events generally were reported early, with higher incidence at ≤180 d (n=364) vs 181-360 d (n=118) or ≥361 d (n=56), and no increased incidence of g≥3 events with prolonged exposure, with the exception of diarrhea (all grade [g≥3]): hyperglycemia (49.2% [31.6%] / 21.2% [11.0%] / 19.6% [10.7%]), hypertension (37.4% [27.2%] / 21.2% [15.3%] / 21.4% [17.9%]), diarrhea (32.7% [3.0%] / 20.3% [4.2%] / 30.4% [12.5%]), nausea (27.5% [1.4%] / 0.8% [0%] / 3.6% [0%]), fatigue (24.2% [2.5%] / 5.9% [0.8%] / 10.7% [1.8%]), pyrexia (19.2% [2.7%] / 16.1% [0.8%] / 10.7% [0%]), and neutropenia (19.5% [16.2%] / 13.6% [8.5%] / 21.4% [16.1%]). A similar trend was observed in pts treated for >1 year (Table), though these pts had a broad range of exposures, with 22 pts treated for ≥2 years. Incidences of g≥3 diarrhea in pts treated for >1 year remained stable and were comparable with prolonged exposure (≤180 d 8.9%; 181-360 d 7.1%; ≥361 d 12.5%). Pneumonitis was infrequent overall (all grade / g≥3: 5.2% / 2.7%) and in pts treated for >1 year (10.7% / 3.6%). Copanlisib-related TEAEs of all grades / g3 / g4 were reported for 88.7% / 54.1% / 18.4% of pts; most events were reported early (≤180 d) with generally no increase in incidence or severity observed in pts treated for >1 year. Serious AEs (SAEs) occurred in 57.1% of pts, most commonly (≥2%; all grade / g≥3) pneumonia (6.0% / 5.5%), general physical health deterioration (5.2% / 4.9%), pyrexia (4.1% / 1.4%), hyperglycemia (4.1% / 4.1%), pneumonitis (4.1% / 2.7%), lung infection (3.0% / 2.2%), and febrile neutropenia (2.2% / 2.2%). All-grade / g3 / g4 copanlisib-related SAEs were reported for 30.2% / 18.7% / 5.8% of pts. As with TEAEs, SAEs mostly occurred early with no general increase in incidence or severity with treatment >1 year. Thirty-nine pts died either during copanlisib treatment or within 35 d post discontinuation of treatment (10.7%), most commonly due to PD (4.7%). No g4 late-onset colitis, hepatotoxicity, or other intestinal toxicity occurred after 6 months of treatment.
Conclusions: These data provide evidence for the manageable safety profile of long-term copanlisib treatment, with no late-onset toxicities or worsening of severity of TEAEs, and few severe gastrointestinal TEAEs. Transient, manageable hyperglycemia and hypertension were among the most common TEAEs; no new unexpected safety issues were identified. These results support the known safety profile and approved indication of copanlisib.
Zinzani:IMMUNE DESIGN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANOFI: Consultancy; CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PORTOLA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; VERASTEM: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Santoro:Gilead: Consultancy, Speakers Bureau; Servier: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; Takeda: Speakers Bureau; Abb-Vie: Speakers Bureau; Sandoz: Speakers Bureau; Arqule: Consultancy, Speakers Bureau; Lilly: Speakers Bureau; MSD: Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Amgen: Speakers Bureau; Celgene: Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Eisai: Consultancy, Speakers Bureau; Novartis: Speakers Bureau; BMS: Consultancy; Roche: Speakers Bureau. Leppä:Roche: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Follows:AZ: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Lenz:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Agios: Research Funding; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding; Roche: Employment, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy. Kim:Celltrion: Research Funding; Mundipharma: Research Funding; J&J: Research Funding; Roche: Research Funding; Kyowa-Kirin: Research Funding; Novartis: Research Funding; Donga: Research Funding. Panayiotidis:Bayer: Other: Support of clinical trial. Demeter:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol- Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Angelini: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Morschhauser:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Munoz:Fosunkite: Speakers Bureau; AstraZeneca: Speakers Bureau; Pharmacyclics LLC an AbbVie Company: Consultancy, Research Funding, Speakers Bureau; Kite Pharma: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Kyowa: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Portola: Research Funding; Incyte: Research Funding. Miriyala:Bayer HealthCare Pharmaceuticals, Inc.: Employment. Benson:Bayer HealthCare Pharmaceuticals, Inc.: Employment. Garcia-Vargas:Bayer Healthcare Pharmaceuticals, Inc.: Employment. Childs:Bayer Healthcare Pharmaceuticals, Inc.: Employment. Dreyling:Novartis: Other: Scientific advisory board; Celgene: Other: Scientific advisory board, Research Funding, Speakers Bureau; Gilead: Other: Scientific advisory board, Speakers Bureau; Janssen: Other: Scientific advisory board, Research Funding, Speakers Bureau; Mundipharma: Other: Scientific advisory board, Research Funding; Roche: Other: Scientific advisory board, Research Funding, Speakers Bureau; Sandoz: Other: Scientific advisory board; Acerta: Other: Scientific advisory board; Bayer: Other: Scientific advisory board, Speakers Bureau.
Copanlisib is approved in the U.S. for the treatment of adult patients with relapsed follicular lymphoma (FL) who have received at least two prior systemic therapies and received U.S. FDA Breakthrough Designation in May 2019 for the treatment of adult patients with relapsed marginal zone lymphoma (MZL) who have received at least 2 prior therapies. We present here Phase I and II clinical trial data of patients with a range of hematological malignancies, including FL and MZL, treated with copanlisib.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal