Background
Hairy cell leukemia (HCL) is a rare and indolent lymphoproliferative disorder. Frontline treatment with a single course of the purine nucleoside analog (PNA) cladribine (or pentostatin) produces a high rate of complete remission (CR) with prolonged durations. At the time of relapse, treatment guidelines recommend re-treatment with a PNA alone or in combination with rituximab (R) but practice patterns vary and data supporting each approach are limited.
Methods
We conducted a multi-site outcomes analysis of patients treated for HCL between 1995-2018 at 5 US medical centers. All patients were treated with frontline PNA and subsequently required treatment for relapsed/refractory (r/r) disease with a PNA alone (PNA) or a PNA with R (PNA+R). Decision to use R was made by the treating physician. Baseline characteristics were compared between treatment cohorts using Fisher's exact and Wilcoxon rank sum tests. Overall survival (OS) and progression free survival (PFS) used log rank test.
Results
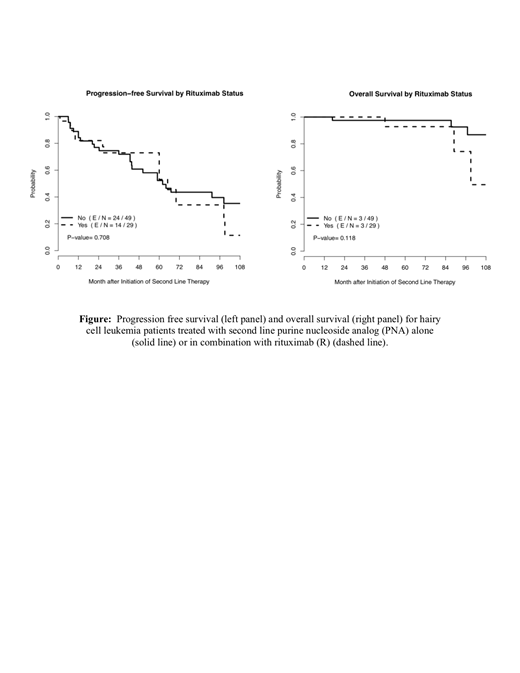
Of the 78 patients analyzed, 49 (62.8%) received a PNA (44 with cladribine and 5 with pentostatin) and 29 (37.2%) received a PNA+R (25 with cladribine and 4 with pentostatin) as second line treatment for r/r HCL. Among the PNA+R cohort, the median number of R doses was 8 (range 1-8). R was administered concurrently in 35% and sequentially in 65% of PNA+R treated patients (N=20 with available data). Baseline characteristics were similar between the PNA and PNA+R cohorts. The median age at diagnosis in the PNA cohort was 52 yo (range 49-87) and 49 yo (range 30-79) in the PNA+R cohort (P=0.62). In the PNA cohort, 36% of patients were female and 64% male vs. 40% and 60%, respectively in the PNA+R cohort (P=0.79). There was no difference in the median time from initial treatment to relapse between the PNA vs. PNA+R cohort [59 months (4-273) vs 61 months (1-99), P=0.29]. At the time of relapse, age, hemoglobin, WBC, ANC, platelet count, and LDH showed no statistical difference between cohorts. Median follow-up for living patients was 57 months (1-197). Median time to best response after second line therapy was 2.4 months (1.0-30.0) in the PNA cohort and 1.2 months (1.0-11) in the PNA+R cohort (P=0.12). Among cases with known responses after second line treatment (N = 50), there was no difference in CR or PR for the PNA vs PNA+R cohorts (93% and 3.4% vs. 95% and 0%, respectively, P = 0.99). Six patients died, 3 from each cohort. Cause of death was due to septic shock in 1 patient and unknown in 5. As shown in the accompanying Figure, there was no difference in median PFS [62 months (95% CI 43.8-not reached) vs. 65 months (95% CI 60-not reached)] or 5-year OS [98% (95% CI 0.93-1) vs. 93% (95% CI 0.8-1), P=0.118] in the PNA vs. PNA+R cohorts, respectively.
Conclusion
Patients with r/r HCL after PNA frontline treatment who receive second line PNA or PNA+R have similar PFS and excellent OS. To our knowledge, this is the largest study evaluating the role of R in treatment of r/r HCL and suggests that there is no clinically discernable advantage to the addition of R to PNA therapy at the time of first re-treatment. The results of this study should be interpreted within the context of a recently reported prospective trial demonstrating improved minimal residual disease-free state with concurrent vs. sequential R with PNA in treatment naïve HCL patients (Chihara, et al. JCO, 37, 2019 (suppl; abstr 7003)).
Pinilla Ibarz:Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Sanofi: Speakers Bureau; Teva: Consultancy; Bayer: Speakers Bureau; TG Therapeutics: Consultancy. Mato:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Celgene: Consultancy; Johnson & Johnson: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member , Research Funding; LOXO: Consultancy, Research Funding; DTRM Biopharma: Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead: Research Funding; Acerta: Consultancy; Janssen: Consultancy. Shadman:Sunesis: Research Funding; AbbVie: Consultancy, Research Funding; Atara Biotherapeutics: Consultancy; Sound Biologics: Consultancy; Celgene: Research Funding; Genentech: Consultancy, Research Funding; BeiGene: Research Funding; Astra Zeneca: Consultancy; Mustang Bio: Research Funding; Acerta Pharma: Research Funding; TG Therapeutic: Research Funding; ADC Therapeutics: Consultancy; Verastem: Consultancy; Pharmacyclics: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Brander:Genentech: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Research Funding; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; BeiGene: Research Funding; DTRM Biopharma: Research Funding; MEI: Research Funding; Acerta: Research Funding; Tolero: Research Funding. Park:Allogene: Consultancy; Amgen: Consultancy; AstraZeneca: Consultancy; Autolus: Consultancy; GSK: Consultancy; Incyte: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Tallman:Biosight: Research Funding; Nohla: Consultancy, Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncolyze: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; KAHR: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; BioLineRx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Cellerant: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetraphase: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hill:Seattle Genetics: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Takeda: Research Funding; TG therapeutics: Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria; Celegene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal