Introduction
Agents targeting BTK have demonstrated activity in a variety of B-cell malignancies, however not all patients respond to therapy, and amongst those that do respond, complete remissions are rare. BTK-based combination regimens have the potential to increase depth of response and permit time-limited therapy, however innovative approaches to accelerate the development of combination regimens are needed. TG-1701 is a novel, orally available and covalently-bound BTK inhibitor that exhibits superior selectivity for BTK compared with ibrutinib in in vitro whole kinome screening (Abstr 3973, EHA 2018). Herein we report the first results of a unique Phase 1 parallel dose-escalation study of TG-1701 monotherapy and TG-1701 in combination with umbralisib, a novel PI3K-δ and casein kinase-1ε inhibitor, and ublituximab, a glycoengineered anti-CD20 mAb (1701 + U2).
Methods
The primary objectives of the study are to characterize the safety profile and to determine the recommended Phase 2 dose of TG-1701 as a single agent and in combination with U2. Other objectives include assessment of pharmacokinetics (PK), preliminary antitumor activity, and pharmacodynamics (PD [BTK occupancy]). Eligible patients must have B-cell malignancy relapsed or refractory (R/R) to one or more prior standard therapy. Treatment consists of escalating doses of oral TG-1701 once daily (QD), continuously administered in 28-day (D) cycles (C). Patients in the 1701 + U2 combination arm receive escalating TG-1701 oral QD + umbralisib 800 mg oral QD + ublituximab 900 mg IV on D1, 8, 15 of C1, and D1 of C2-6. All patients are treated until disease progression, unacceptable toxicity, or investigator/patient decision to withdraw study consent.
Results
As of July 15, 2019, 19 patients (WM = 6, CLL = 4, FL = 4, MZL = 2, DLBCL = 2, MCL = 1) have been treated with TG-1701: 3 patients at 100 mg QD, 9 patients at 200 mg QD (expansion before opening combination), 3 patients at 300 mg QD single agent arm, and 4 patients at 100 mg QD combination arm. Patients had a median of 2 prior systemic therapies (range, 1 - 5), and all of them received previous anti-CD20 therapy. Four patients were refractory to their last prior therapy, 8 had extranodal disease, and 8 had bulky disease. To date, patients have received a median of 4 (range, 2 - 10) cycles of TG-1701.
No dose-limiting toxicity (DLT) have been observed to date. The most common treatment-related adverse events (TRAE) are: neutropenia (21%), diarrhea (16%), nausea (16%), bruising (16%), infection (16%), ALT/AST elevation (11%), rash (11%), abdominal pain (11%), and fatigue (11%). Grade 3 TRAE are: asymptomatic and isolated lipase elevation (N=1), nausea (N=1), rash (N=1), and acute respiratory tract infection (N=1). There have been no grade 4 TRAE nor treatment discontinuations due to adverse events.
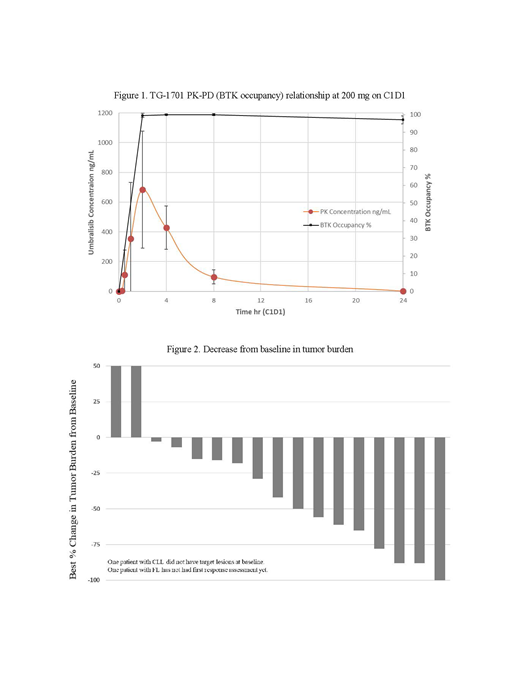
Linear kinetics are apparent, evidenced by approximately dose proportional increase in AUC on C1D1 and C1D8. High systemic clearance (CL) has been observed with a mean CL/F of 55.4 L/hr and half-life of 2.24 hours. Tmax is observed between 1 to 4 hours post dose. PK-PD (BTK occupancy) relationship at 200 mg on C1D1 is presented in Figure 1. Decrease from baseline in tumor burden is presented in Figure 2. Two patients at the lowest dose (100 mg QD) have achieved a partial response (PR): MCL = ↓78% and WM = ↓88%. All 3 evaluable patients treated with 100 mg 1701 + U2 have achieved a response at the first response assessment: 1 CR (FL) and 2 PR (FL ↓88%, and MZL ↓65%). All patients remain on study treatment, except for both patients with DLBCL that discontinued due to disease progression. All patients with CLL have shown lymphocytosis during the first 2 cycles of therapy. Near complete BTK occupancy has been achieved in all patients at all dose levels (Figure 1).
Conclusions
TG-1701, a once daily BTK inhibitor has an encouraging preliminary safety profile, with clinical and pharmacodynamic activity at all dose levels evaluated. This study (NCT03671590) continues enrollment in TG-1701 single-agent and 1701 + U2 combination arms.
Cheah:Roche, Janssen, MSD, Gilead, Loxo Oncology, Acerta, BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene, Roche, Abbvie: Research Funding; Roche: Other: Travel expenses. Wickham:Roche: Honoraria; Celgene: Other: Travel/Meeting Expenses . Turpuseema:TG Therapeutics Inc.: Employment, Equity Ownership. Miskin:TG therapeutics Inc.: Employment, Equity Ownership. Tang:TG Therapeutics Inc., Roche, Alexion: Equity Ownership; TG Therapeutics Inc.: Employment. Normant:TG Therapeutics Inc.: Employment, Equity Ownership. Ricart:TG Therapeutics, Pfizer, Merck: Equity Ownership; TG therapeutics Inc.: Employment. Tam:Abbvie, Janssen, Beigene, Roche, Novartis: Honoraria; Abbvie, Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal