Background: Early events within 24 months (POD24) of initial immunochemotherapy (IC) were repeatedly confirmed to be associated with poor survival in follicular lymphoma (FL). Rituximab maintenance (RM) given to responding patients after IC is effective strategy to reduce relapse incidence and improve survival (Salles, Lancet 2011; Hill, BJH 2019). There is little known about the POD24 incidence pattern in chemosensitive patients, and the impact of subsequent RM application for POD24 reduction has not been studied yet.
Aim: To (1) analyze the role of POD24 events in patients with responding FL and (2) describe whether post-induction RM changes the pattern of POD24 incidence and possibly post-POD24 outcome of the patients.
Methods: The study comprised patients prospectively enrolled in the Czech Lymphoma Study Group network (ClinicalTrials.gov: NCT03199066) in 2000-2015, with grade 1-3A FL and treated with frontline R-CHOP/R-CHOP-like or R-bendamustine or R-CVP IC. Cases with previous WaW were not enrolled. No frontline stem cell (auto/allo) transplant was allowed. Only patients who achieved complete (CR/CRu) or partial remission (PR) at the end of induction (EOI) and were thus potential candidates for RM were included. Early event was defined as progression, relapse or death within 24 months after the date of EOI response assessment. Overall (OS) and progression-free survival (PFS) were calculated from the date of diagnosis and date of EOI response (OS-EOI). Patients who experienced an event before the median time to RM initiation were excluded from POD24 survival analyses to avoid overestimation of the RM group results.
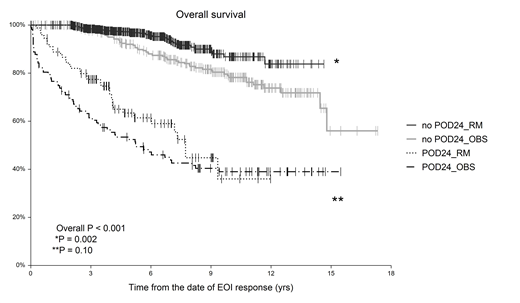
Results: A total of 1089 patients were identified, of whom 729 (67%) received RM (maintenance group) and 360 (33%) were followed without RM (observation group; OBS). When comparing both groups, we found no differences in age (median age 59 yrs for both; P=0.54), sex distribution (males 39% vs. 41% in OBS vs. RM, respectively; P=0.37) and ECOG 0-1 (90% vs. 93% in OBS vs. RM; P=0.10). There was a slightly lower proportion of advanced FL (Ann Arbor III-IV) in OBS (80%) as compared to RM (87%, P=0.01), which translated into a lower proportion of high FLIPI patients (46% vs. 52% in OBS vs. RM, respectively; P=0.003). The induction regimens were as follows: R-CHOP in 70% and 83%, R-COP in 18.6% and 10.7%, R-bendamustine 1% and 4%, R-fludarabine-based in 4% and 1% and others in 5% and 1% in RM and OBS, respectively. The EOI remission status was assessed 61 days (median) after the last IC dose with CR/CRu/PR rates of 65%/9%/26% and 65%/6%/29% in OBS and RM, respectively (P=0.14). The median time from EOI response to the first RM dose was 48 days (range 0-371 days), 89% of the pts have started RM within 120 days. The median number of RM doses given was 8 (range, 1-36), with 85% of patients receiving 8-12 doses of RM. After a median follow-up of 10.4 yrs (OBS) vs. 6.0 yrs (RM); P<0.001, 365 (33.5%) patients progressed or relapsed and 177 (16.3%) died. The 5-year OS and PFS were 84.2% vs. 93.6% (P<0.001) and 54.1% vs. 73.6% (P<0.001) in OBS and RM, respectively. The cumulative incidence of POD24 was higher in OBS (29.7%) than in RM (12.3%; P<0.01). POD24 cumulative incidence correlates with depth of EOI response with 21.8%/35.5%/50.5% and 8.2%/14.6%/21.1% for CR/CRu/PR categories in the OBS and RM groups, respectively. When combining RM application (yes/no) and POD24 incidence (yes/no), survival analysis stratified patients into 4 subgroups with 5-year OS-EOI of 52.4%, 63.4%, 92.0% and 96.9% for POD24-OBS, POD24-RM, noPOD24-OBS and noPOD24-RM patients, respectively (P<0.001); see Figure 1. Patients who developed POD24 on RM had only a 26% lower risk of death, compared to OBS (HR 0.74; P=0.15). POD24-free patients experienced a 50% death reduction after RM (HR 0.49; P=0.002).
Conclusion: Early progression of the disease significantly increases the risk of death even in pts who respond well to the initial IC. Application of RM significantly prolonged both OS and PFS and reduced POD-24 incidence by half. However, once patients developed an early event on RM, their outcome remained poor. On the other hand, in the POD-24-free group, RM brought survival benefit that lasted beyond its termination. Large independent validation may enhance the validity of these data since the observation group was mainly of a historical nature.
Acknowledgement: Supported by IGA_LF_2019_001, MH CZ - DRO (FNOL, 00098892), and AZV 16-31092A grants.
Prochazka:Roche: Consultancy; Takeda: Research Funding. Trneny:Morphosys: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Gilead sciences: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal