
Introduction: Treatment of AML remains challenging. Decitabine is a hypomethylating agent (HMA) commonly used for treating AML. However, its efficacy is limited. Recent success targeting immune checkpoints offers great promise and several agents blocking the PD-1 pathway have been FDA approved for cancer therapy. It has been demonstrated that HMA enhances PD-1 pathway in MDS and AML patients, providing a strong rationale for combining HMA and PD-1 inhibition in AML treatment. Avelumab is a PD-L1 antibody that blocks the interaction between PD-L1 and PD-1. It has been FDA approved for treating Merkel cell and urothelial carcinoma and in combination with axitinib for renal cell carcinoma. This phase I study aimed to investigate the safety of avelumab combining with decitabine in AML. In addition, extensive correlative studies were performed to define the effect of avelumab on the immune response.
Methods: This is a single arm, open label phase I study to evaluate safety and tolerability of avelumab in combination with decitabine in patients with untreated AML, who are unfit for intensive chemotherapy. Decitabine was given at 20mg/m2 IV day 1-5, every 28 days. Avelumab was given at 10mg/kg IV day 1, every 14 days. Each treatment cycle was 28 days. Treatment length was up to one year or until progression. In the initial stage, classic "3+3" design was applied to determine safety. Patients were evaluated for DLTs in cycle 1. In the expansion stage, a cohort of 9 additional patients using the same regimen was planned. For correlative studies, blood and bone marrow samples were collected prior to and post initiation of avelumab. Flow cytometry-based assays for immune phenotype and functions were performed.
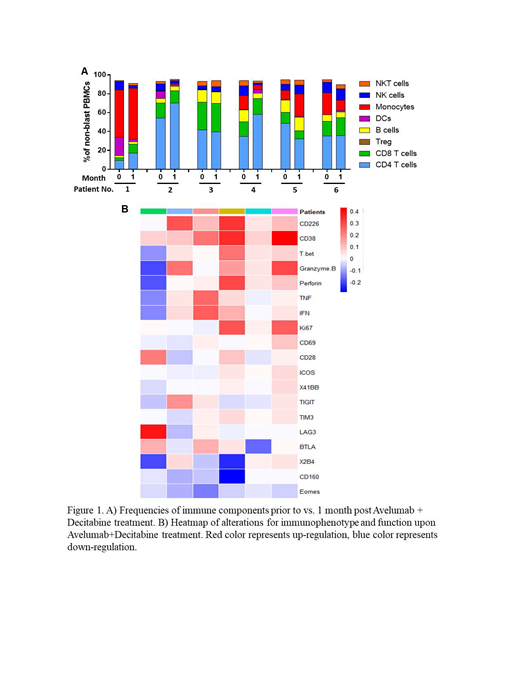
Results: Seven patients have been treated on protocol. Median age was 71 (62-78). Most patients (6/7) carried adverse cytogenetics per ELN 2017 risk stratification. Median number of treatment cycles was 2 (range 1-12). No DLT was detected. The most frequent TEAEs (any grade or cause) were anorexia (86%), fatigue (71%), edema (71%), pleural effusion (71%), febrile neutropenia (57%) and respiratory failure (57%). Two treatment-related TRAEs were observed: fever (14%) and pneumonitis (14%). TRAEs led to treatment discontinuation in 1 patient (14%). The trial was closed before accrual met for the best interest of patients due to the recent FDA approval of venetoclax, a novel treatment for the same patient population. Among the 7 patients accrued, 1 patient achieved CR, 3 patients stable disease, 1 had progression of disease; response could not be assessed in 2 patients due to death before response assessment. To investigate the effect of avelumab on immune response, we conducted complex immune assays using blood samples collected from each patient prior to vs. 1 month post treatment. We observed no alteration in the frequency of each immune component (NK, NKT, B cells, DCs, Monocytes, CD4 T cells, CD8 T cells, and Treg) upon avelumab and decitabine treatment. However, there was an increase in effector memory differentiation while decreased terminal differentiated subsets in CD8 T cells. We next performed comprehensive phenotypic and functional analysis of CD8 T cells. Data of the first accrued 6 patients are shown here (Figure 1); the data from patient 7 is underway and will be integrated into the presentation at the meeting. We observed a strong trend of up-regulation of activation markers and co-stimulatory receptors (CD69, CD226, CD28, ICOS and 4-1BB) on CD8 T cells post treatment of avelumab and decitabine. In contrast, the expression of inhibitory molecules, including TIGIT, TIM-3, CD160, LAG-3, 2B4 and BTLA, were trending down. Consistently, CD8 T cell function was enhanced in majority of patients, manifested by higher expression of granzyme B, perforin and Ki67, as well as more cytokine release (IFN-γ and TNF-α) upon in vitro TCR engagement. Importantly, avelumab is likely the major contributor for the positive regulatory effect as studies on samples from patients who received decitabine alone did not show the same trend.
Conclusion: In this phase I study, we demonstrated that avelumab in combination with decitabine as first line treatment of unfit patients with AML is safe and tolerable. CD8 T cell response trends up upon avelumab treatment. Our study strongly supports the addition of avelumab to AML treatment with a potential to improve anti-leukemia immunity.
Zheng:Pfizer: Research Funding. Claxton:Daiichi Sankyo Co. and Ambit Biosciences Corp, Astellas Pharma, Novartis Pharmaceuticals, Incyte Corporation, Cyclacel Pharmaceuticals, Inc, Celegene Corporation, Medimmune, Inc, Merck Sharp & Dohme Corp., Gilead Sciences, Inc.: Research Funding. Naik:Celgene: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal