Objectives: Over the last 50 years, there has been a steady improvement in the treatment outcome of AML. However, median survival in the elderly is still poor due to intolerance to intensive chemotherapy and higher numbers of patients with adverse cytogenetics. CYC065, a novel CDK inhibitor, has pre-clinical efficacy in AML. The survival of AML blasts is dependent on MCL-1 which is depleted following treatment with CYC065. The aims of this study are: i) to demonstrate target inhibition and characterise the mechanism of action of CYC065 in vitro; ii) to assess the effects of CYC065 on growth and survival of AML cells; and iii) to assess a synergistic effect of CYC065 in combination with other targeted or chemotherapy agents on growth and survival of AML cell lines and primary human AML cells.
Methods: Western blotting was performed to elucidate the mechanism of action. CYC065 effects on gene expression were studied in three AML cell lines: OCI-AML3 (NPM1 and DNMT3A mutations), MOLM-13 (MLL-AF9) and MV4-11 (FLT3-ITD, MLL-AF4) by qRT-PCR using Fluidigm® Biomark technology. The efficacy of CYC065 was explored in the three cell lines in parallel with washout studies. A synergistic effect of CYC065 in combination with venetoclax, cytarabine, or azacitidine was explored in the three AML cell lines, seven primary AML and three normal control samples using a variety of assays measuring cell viability, apoptosis and proliferation.
Results: Inhibition of CDK9 by 1µM CYC065 treatment for 4h and 24h was demonstrated in AML cell lines by a reduction in phosphorylation of pSer2 RNA polymerase II, leading to inhibition of transcription and loss of transcripts with short half-lives. As expected from the proposed mechanism of action, gene expression was generally suppressed following CYC065 treatment. Early events (4h) included decreases in cell cycle regulators including CDK7 and CDK9, E2F1, CDC25C and PPP1R10, pro-survival molecules, including MCL-1, BCL-2 and XIAP, and the MLL target genes, MEIS1 and RUNX1. Decreases in MCL-1 mRNA were confirmed at the protein level by Western blotting and preceded the induction of apoptosis (PARP cleavage). In functional assays, clinically relevant CYC065 concentrations of 0.75µM, 0.5µM and 1µM, which induce 50% apoptosis, were selected for the OCI-AML3, MOLM-13 and MV4-11 cell lines, respectively, to combine with 0.0018-0.9µM of venetoclax, 0.016-0.47µM of cytarabine, or 2-5.3µM of azacitidine using the drug combination ratio obtained from synergy assays using CompuSyn software. Preliminary results showed a synergistic activity in all cell lines. Washout studies showed a slight recovery of cell viability at low concentrations but not at 1μM CYC065.
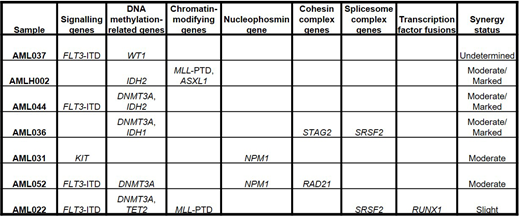
High diversity of genetic mutations was observed in the seven primary AML samples (see Table). In general, moderate to marked synergistic effects were observed in apoptosis and active caspase-3 assays when CYC065 in combination with the three partners when clinically achievable concentrations were used. The more complex the molecular genetic lesions or complexity of karyotype, the less efficacious the combination therapy. In proliferation assays, CYC065/venetoclax showed a slight synergistic effect, whereas, CYC065/cytarabine and CYC065/azacitidine combinations showed a marked synergistic effect in which an increase in a percentage of cell division arrest was observed.
Conclusions: CYC065 pulse treatment effectively induced apoptosis in AML cell lines in vitro. Target inhibition was confirmed by analysis of gene and protein expression and was accompanied by induction of apoptosis and cell cycle arrest. A synergistic effect of CYC065 in combination with venetoclax, cytarabine, or azacitidine was seen in AML cell lines and primary AML cells. These results highlight the potential of CYC065 in combination with venetoclax or standard chemotherapy agents for the treatment of AML.
Zheleva:Cyclacel Ltd: Employment, Equity Ownership, Patents & Royalties. Copland:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Speakers Bureau; Cyclacel: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal