
Hypomethylating agents (HMAs) are standard first line therapy for patients with high risk MDS and older patients with AML. Outcomes for patients who do not respond or progress on HMAs are poor, and there are no standard treatment options for these patients. The interaction between leukemic blasts and the bone marrow microenvironment is thought to play an important role in disease pathogenesis and possibly chemotherapy resistance, so we hypothesized that blocking the CXCL12/CXCR4 axis with CX-01 may improve response rates to azacitidine in patients who have failed HMA therapy. CX-01 is a low molecular weight heparin derivative with minimal anticoagulant activity that binds CXCL12 as well as neutralizes PF4, a negative regulator of megakaryopoiesis.
We report updated study results of a single institution a pilot study (NCT02995655) that enrolled patients with HMA-refractory (received 4 or more cycles of HMA without response or disease progression on HMA therapy) AML or MDS (INT-1 or greater). Patients received 7 day continuous infusion of CX-01 (0.25 mg/kg/hr) and azacitidine 75 mg/m2 daily days 1-7, in 28 day cycles. The primary objective of this trial was to assess the overall response rate (ORR).
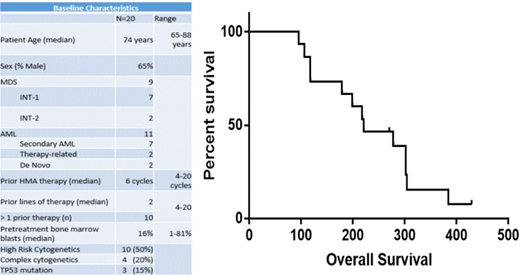
20 patients were enrolled between May 2017 and February 2018. The median age was 74 years (range 62-88 years) and 35% were female. Nine patients had MDS and 11 had AML, with 7 patients having antecedent MDS and 2 having therapy-related AML. Half of the patients had high risk cytogenetic abnormalities and 3 had TP53 mutations. Patients had a median of 2 prior lines of therapy (range 1-3) with median of 6 prior cycles of HMA therapy (range 4-20). Only 4 patients had a confirmed response to prior HMA therapy.
Of the 20 patients that were enrolled, 15 were considered evaluable for response with a bone marrow biopsy after cycle 2. Of the 5 unevaluable patients, 3 were removed from the trial before completing C1 due to transaminitis, discovery of a second primary malignancy, and an elevated PTT on day 2. One patient had grade 5 sepsis during C1, and another withdrew for unrelated reasons. As expected with azacitidine therapy in this patient population, hematologic toxicity was common with 16 instances of grade 3/4 neutropenia/leukopenia, 5 instances of grade 3/4 anemia, and 10 instances of grade 3/4 thrombocytopenia. Other common grade 3/4 adverse events were various infections (20), febrile neutropenia (13), hypertension (10), electrolyte disturbance (9), and transaminitis (3). Mild prolongations in PTT (22) and INR (7) were common, and there was one instance of grade 3 PTT prolongation, two instances of grade 3 epistaxis, and one instance of grade 3 hematuria. The incidences of bleeding were not thought related to CX-01 infusion.
The 15 evaluable patients received a median of 3 cycles of CX-01 and azacitidine (range 2-9). Of 15 evaluable patients, there was 1 CR and 3 bone marrow CRs (mCR, with incomplete blood count recovery), 9 stable disease, and 2 progressive disease for an ORR of 27%. Of the 3 patients with a mCR after cycle 2, two had hematologic improvement of their neutrophil and platelet counts, respectively, by the end of cycle 4. A patient with stable disease also had hematologic improvement in platelets.
The median OS of evaluable patients was 221 days (95% CI 179 days-not reached) and median overall survival was not significantly different between AML patients at 221 days and MDS patients at 248 days (p = 0.8).
In this pilot study we demonstrated the feasibility of treating HMA-refractory AML and MDS with CX-01 and azacitidine. While this study is limited by its small sample size, we observed higher than expected response rate and favorable OS compared to historical controls.
Cashen:Celgene: Other: Speaker's Bureau; Seattle Genetics: Other: Speaker's Bureau; Novartis: Other: Speaker's Bureau. DiPersio:Incyte: Consultancy, Research Funding; WUGEN: Equity Ownership, Patents & Royalties, Research Funding; Magenta Therapeutics: Equity Ownership; Amphivena Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; RiverVest Venture Partners Arch Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellworks Group, Inc.: Membership on an entity's Board of Directors or advisory committees; NeoImmune Tech: Research Funding; Macrogenics: Research Funding, Speakers Bureau; Bioline Rx: Research Funding, Speakers Bureau; Karyopharm Therapeutics: Consultancy. Jacoby:Celgene: Speakers Bureau; Novo Nordisk: Consultancy; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees. Uy:Astellas: Consultancy; Pfizer: Consultancy; Curis: Consultancy; GlycoMimetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal