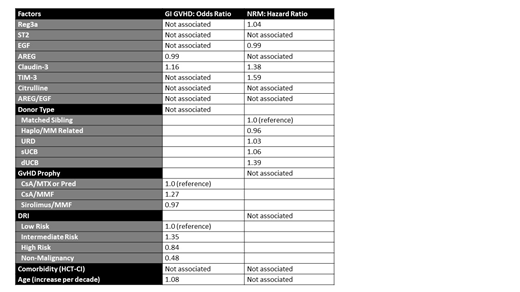
Intestinal damage from prior chemotherapy or infections can contribute to the risk of gastrointestinal acute graft-versus-host disease (GI GVHD) and non-relapse mortality (NRM) after allogeneic hematopoietic cell transplantation (HCT). However, identifying patients who appear healthy but are at increased risk of GI GVHD and NRM due to pre-transplant intestinal tissue damage is challenging. The primary objective of this study was to determine the independent association of serum pre-transplant biomarkers associated with intestinal damage and GI GVHD on the incidence of GI GVHD and 1-year NRM. Specifically, we analyzed amphiregulin (AREG), epidermal growth factor (EGF), ratio of AREG/EGF, regenerating islet-derived 3a (REG3a), suppressor of tumorigenicity 2 (ST2), claudin-3, citrulline, and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) in pre-transplant serum samples from 528 unique pediatric and adult (median age 41, range <1-75 years) patients undergoing first allogeneic HCT at the University of Minnesota between 2010 - 2018. To identify the most influential predictors, we performed lasso (least absolute shrinkage and selection operator) regression (logistic for GI aGVHD and Cox for NRM), rather than standard regression, which too often results in model overfitting. We included standard clinical factors as potential confounders, including patient age (per increase by decade), donor type (matched sibling vs. haploidentical/mismatch related, unrelated donor [URD] vs. single umbilical cord blood [sUCB] vs. double umbilical cord blood [dUCB]), underlying disease risk (low disease risk vs. intermediate disease risk vs. high disease risk vs. non-malignancy), GVHD prophylaxis (CsA or TAC/MTX or Prednisone vs. CsA or TAC/MMF vs. Sirolimus/MMF) and comorbidity (HCT-CI; low risk vs. intermediate risk vs. high risk). In pre-transplant serum samples, only claudin-3 was associated with a risk of subsequent GI GVHD (odds ratio [OR] 1.16). In this cohort, the median pre-transplant claudin-3 was 29.4 pg/ml (range 5.1 - 120.6 pg/ml). The optimal cutpoint for subsequent GI GVHD was 24.5 pg/ml. Nearly one-third of patients with claudin-3 at or above this level developed GI GVHD (31%, 95% CI 13-26%), compared to 19% of patients with a claudin-3 <24.5 pg/ml developing GI GVHD (95% CI 13-26%). Pre-transplant claudin-3 was also associated with NRM, with an optimal cutpoint of 27.1 pg/ml and twice the proportion of 1-year NRM above that cutpoint (24%, 95% CI 19-28% vs 12%, 95% CI 8-17%). Higher REG3a (OR 1.04) and TIM-3 (OR 1.59) were also associated with NRM, whereas higher pre-transplant EGF was protective against NRM (OR 0.99) and AREG protective against GI GVHD (OR 0.99). Claudin-3 was not strongly correlated with the other biomarkers and was lower in patients with non-malignant disease compared to patients undergoing transplant hematologic malignancies (median 26.3 vs 30.4-32 pg/ml, p<0.01). We conclude that claudin-3, a major component of tight junctions between epithelial and endothelial cells, is the most promising pre-transplant biomarker of increased GI GVHD and NRM risk among those tested. Patients with elevated pre-transplant claudin-3 serum levels may have organ tissues less resilient to damage from chemotherapy, radiation, and infections and could thus be appropriate for studies of novel toxicity-sparing approaches.
Holtan:Bristol-Myers Squibb: Consultancy; Incyte: Consultancy; Janssen: Consultancy; CSL Behring: Consultancy. Weisdorf:Fate Therapeutics: Consultancy; Pharmacyclics: Consultancy; Incyte: Research Funding. MacMillan:Angiocrine: Membership on an entity's Board of Directors or advisory committees; Equillium: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Alpine Immune Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal