Background:
Patients with acute myeloid leukemia (AML) with myelodysplasia related changes (MRC) or therapy related myeloid neoplasm have poorer outcomes compared to patients with de novo AML. CPX-351 was approved by the Food and Drug Administration (FDA) in 2017 as an induction chemotherapy regimen for patients with AML-MRC or t-AML as defined by 2016 World Health Organization (WHO) classification. A large randomized phase 3 study that led to approval of CPX-351 included only the patients between age 60 and 75. However, the FDA approval extends to patients of age ≥18 years with limited data regarding its efficacy in a younger patient population.
Methods:
We performed a retrospective study of clinical and molecular data of 89 patients treated at Moffitt Cancer Center with CPX-351 as frontline induction chemotherapy regimen in patients with AML. Clinical data was abstracted in accordance with institutional review board approved protocol. Patients were then divided in two subgroups: Cohort A) Age <60 years and cohort B) Age ≥60 years. We calculated overall response rate (ORR) defined as the proportion of patients achieving complete remission (CR), CR with incomplete count recovery (CRi) or morphologic leukemia free state (MLFS). We then calculated median overall survival (mOS) using Kaplan Meier method with log-rank test to determine significance. Fisher's Exact method was utilized to determine significance for categorical variables. All reported p-values are two sided.
Results:
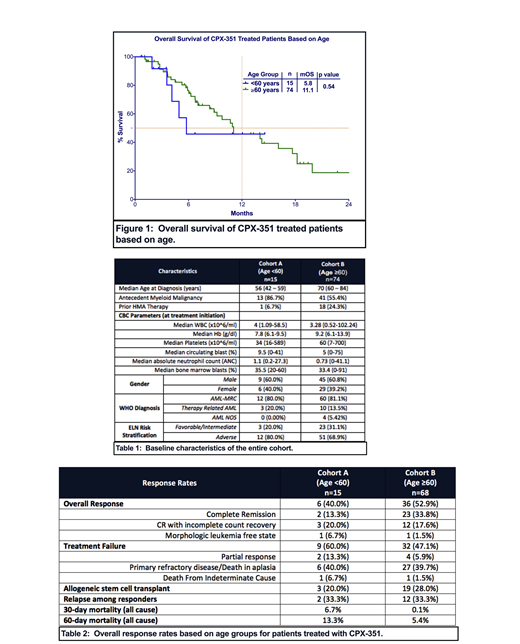
Out of a total 89 patients included in the analysis, 17% (n=15) of patients were in cohort A and 83% (n=74) of patients were in cohort B. In cohort A, 40% of patients were female compared to 39% of patients in cohort B. Median age in cohort A was 56 years old (range: 42-59) and 70 years old in cohort B (range: 60-84). Overall, 71% of the patients had adverse risk disease as defined by European Leukemia Net (ELN) classification, which includes 80% of patients in cohort A and 69% of patients in cohort B. Baseline characteristics are described in Table 1.
Among 83 patients evaluable for response, overall response rate (ORR) of the entire cohort was 51% (Table 2). ORR in cohort A was 40% compared to 53% in cohort B (p=0.41). ORR in ELN defined adverse risk group was 47% in the entire cohort and was not significantly different between cohort A and B (33% vs. 51%, p=0.34). Overall 30-day mortality rate of the entire cohort was 2.2%, 6.7% in cohort A and 0.1% in cohort B. Overall 60-day mortality rate of the entire cohort was 6.7%, 13.3% in cohort A and 5.4% in cohort B. Overall, 27% of patients proceeded to allogeneic stem cell transplant (allo-SCT), including 20% of patients in cohort A and 28% of patients in cohort B (p=0.75).
We then evaluated ORR according to somatic mutations prior to initiation of therapy. The most common gene mutation was ASXL1 in 28% of patients followed by TET2 (25%) and RUNX1 (19%). Patients with IDH1/2 mutations had superior response rates compared to patients without IDH1/2 mutations (13/16 (81%) vs. 67/142 (47%), p=0.02). Among patients with TP53 mutated disease (n=12), which include 20% of patients in cohort A and 13% of patients in cohort B, ORR was 25% with 17% achieving CR and was not significantly different between cohort A and B (p=1.0).
At median follow up of 13.0 months, mOS of the entire cohort was 11.1 months. Patients in cohort A did not have significantly different mOS to patients in cohort B (5.8 vs. 11.1 months, p=0.54) (Figure 1). Since there was a higher percentage of patients in cohort A with TP53 mutation than patients in cohort B (20% vs. 13%), we estimated mOS in patients without TP53 mutation and mOS was not different between cohorts A and B (13.4 vs. 11 months, p=0.59). Among responding patients, mOS was not reached in cohort A compared to 17.7 months in cohort B (p=0.59).
Conclusion:
We demonstrate that CPX-351 results in similar survival outcomes in both younger and older adults with AML with MRC or therapy-related AML. Our data suggests that CPX-351 is a viable treatment option for any age group where inherent disease biology dictates outcome rather than age.
Padron:Incyte: Research Funding. Kuykendall:Abbvie: Honoraria; Janssen: Consultancy; Incyte: Honoraria, Speakers Bureau; Celgene: Honoraria. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Komrokji:Incyte: Consultancy; DSI: Consultancy; pfizer: Consultancy; celgene: Consultancy; JAZZ: Consultancy; Agios: Consultancy; JAZZ: Speakers Bureau; Novartis: Speakers Bureau. Lancet:Pfizer: Consultancy, Research Funding; Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy; Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services . Sallman:Celyad: Membership on an entity's Board of Directors or advisory committees. Sweet:Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau; Jazz: Speakers Bureau; Incyte: Research Funding; Pfizer: Consultancy; Stemline: Consultancy. Talati:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Daiichi-Sankyo: Honoraria; Astellas: Honoraria, Speakers Bureau; Pfizer: Honoraria; Celgene: Honoraria; Agios: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal