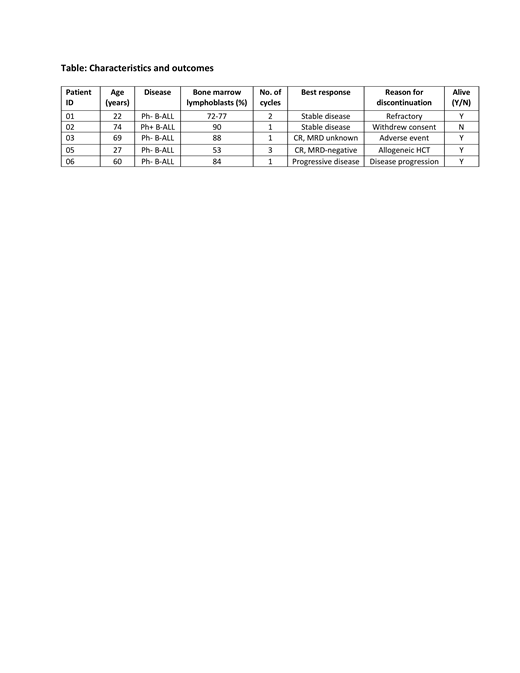
Clinical and preclinical findings suggest that PD-L1 overexpression on lymphoblasts and in the bone marrow microenvironment may mediate resistance to blinatumomab by inhibiting T-cell activation. We report preliminary findings from an ongoing phase I/II multicenter trial to evaluate the safety and efficacy of blinatumomab with pembrolizumab in adults with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and a high bone marrow lymphoblast percentage (NCT 03160079). The primary objective of this Phase I/II trial is to determine overall response rate (ORR = complete response (CR) + complete response with partial hematologic recovery (CRh) rate) after 1-2 cycles of blinatumomab with pembrolizumab, with key secondary endpoints of adverse events (AEs), minimal residual disease (MRD)-negative CR/CRh rate, 2-year disease-free and overall survival, and allogeneic HCT rate. Eligible patients are 18 years of age or older with R/R B-ALL after ≥ 1 prior line of therapy (including Philadelphia chromosome positive (Ph+) B-ALL failing one second or third generation tyrosine kinase inhibitor) and >50% lymphoblasts on screening bone marrow sample. Patients receive blinatumomab by continuous IV at 9 mcg/day on days 1-7 and 28 mcg/day on days 8-28 of cycle 1, then 28 mcg/day on days 1-28 in subsequent cycles. Pembrolizumab 200 mg IV is given on days 15 and 36 of each 42-day cycle. Patients in CR/CRh after 1-2 cycles complete a maximum of 5 cycles. A safety cohort of up to 6 patients assessed safety by 3+3 design. Dose-limiting toxicities (DLTs) were defined as Grade 3 or 4 non-hematologic AEs related to the addition of pembrolizumab to blinatumomab with a DLT monitoring period of 28 days from the first pembrolizumab dose. At the time of this analysis, 5 patients have been enrolled and treated with all 5 completing the DLT monitoring period. Patients had a median age of 60 years (range 22-74) and one had Ph+ disease. Median bone marrow lymphoblast percentage at time of enrollment was 84% (range 53-90). Patients received a median of 1 cycle (range 1-3) of blinatumomab with pembrolizumab. Common AEs included fever, headache, increased bilirubin, nausea, neurotoxicity, and tachycardia. Grade 3-4 non-hematologic AEs included disseminated intravascular coagulation, hyperferritinemia, hypokalemia, subdural hematoma, encephalopathy, hyponatremia, and macrophage activation syndrome in 1 patient (all related to blinatumomab), hyperbilirubinemia and elevated AST in 1 patient, and hypertriglyceridemia in 1 patient. No grade 3 or greater immune-related AEs have occurred. No pembrolizumab-related DLTs occurred in the first 5 patients in the safety cohort and enrollment is now proceeding in the dose-expansion cohort. The ORR was 50% with 2/4 evaluable patients achieving a CR. One patient achieved an MRD-negative CR in cycle 1 and completed 3 cycles before proceeding to allogeneic HCT. One patient discontinued treatment due to subdural hemorrhage and macrophage activation syndrome during cycle 1 and achieved a CR. Both patients remain in CR for over 6 months. Two patients discontinued treatment due to refractory or progressive disease. The one patient not evaluable for response withdrew from study therapy after 1 cycle without ALL progression. Patient, disease, and treatment characteristics as well as outcomes are summarized in the Table. Blinatumomab with pembrolizumab is safe for adults with R/R B-ALL and a high bone marrow lymphoblast percentage. Enrollment continues in the dose-expansion cohort to assess efficacy.
Damon:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Costello:Celgene: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Takeda: Honoraria, Research Funding. Schiller:Biomed Valley Discoveries: Research Funding; Astellas: Research Funding; Amgen: Other, Research Funding; Agios: Research Funding, Speakers Bureau; Bristol Myer Squibb: Research Funding; Celgene: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding. Wieduwilt:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen, Leadiant, Merck, Servier: Research Funding; Reata Pharmaceuticals: Equity Ownership; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees.
Pembrolizumab (given off label)to enhance the efficacy of blinatumomab (given on label) for relaped/refractory B-cell acute lymphoblastic leukemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal