Background: The current success in treating hematological malignancies with CD19 targeted chimeric antigen receptor (CAR) T cells is diminished by tumor relapse from target antigen loss. The B-cell activating factor-receptor (BAFF-R), a tumor necrosis factor receptor superfamily protein (TNFRSF13C), is a particularly interesting alternative target in CD19 relapsed disease and in B cell malignancies in general. BAFF-R null mice exhibit greatly reduced normal B-cell numbers and mouse strains expressing a mutant BAFF-R exhibited decreased B-cell life spans and a dramatically reduced peripheral B-cell compartment. Thus, BAFF-R signaling is a driver of B-cell survival, which may limit the capacity of clonal B-cell tumors to escape therapy by down-regulation of antigen expression.
We have exploited the potential of the BAFF/BAFF-R axis and developed a proprietary BAFF-R targeting CAR T cell using a single-chain variable fragment (scFv) of a humanized anti-human BAFF-R antibody engineered into a second generation CAR construct containing 4-1BB costimulatory and CD3ζ intracellular signaling domains (Qin et al. Sci. Transl. Med., In Press). BAFF-R CAR T cells were cytotoxic against a panel of human leukemia and lymphoma lines. Adoptively transferred BAFF-R-CAR T cells eradicated 10-day pre-established tumor xenografts after a single treatment and retained efficacy against xenografts deficient in CD19 expression, including CD19-negative variants within a background of CD19-positive lymphoma cells. BAFF-R-, but not CD19-CAR T cells, also demonstrated antitumor effects against an additional CD19 antigen loss primary patient-derived xenograft (PDX) in vivo.
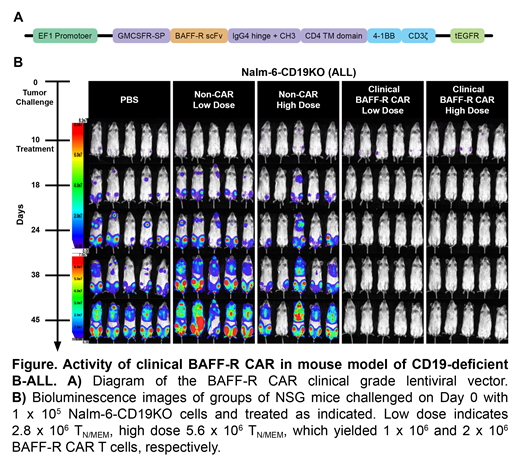
Methods and Results: A first-in-human clinical trial of this BAFF-R CAR T cell in B-ALL is planned. To this end, we cloned the BAFF-R CAR construct into a previously FDA approved clinical lentiviral vector (Figure, part A), and produced BAFF-R CAR T cells using both the research prototype and clinical vectors for head-to-head analysis. We observed comparable cytotoxic T lymphocyte activity against previously established leukemia and lymphoma models in vitro. In vivo, NSG mice challenged with Nalm-6 human B-ALL tumors were randomized (n=5/group) for treatment with either BAFF-R CAR produced from the research prototype or clinical vectors. The two experimental groups were indistinguishable in their antitumor efficacy (data not shown).
Further, to advance IND enabling studies we also compared clinical CAR T cell manufacturing strategies. We used an early stage TN/MEM subset for CAR T cell production, as used in previous CAR T cell trials at our institution, and using TN/MEM isolated from healthy donor PBMCs achieved 36% transduction efficiency, comparable to clinical experience at City of Hope.
Finally, we assessed the therapeutic effects of BAFF-R CAR TN/MEM cells in vivo. We challenged NSG mice on day 0 with 1 x 105 Nalm-6-CD19 deficient (Nalm-6-CD19KO) human ALL tumor cells and randomized them (n=5/group) to receive a single infusion of either low dose (1 x 106) or high dose (2 x 106) BAFF-R-CAR TN/MEM cells on day 10. Because transduction efficiency was 36%, 2.8 or 5.6 x 106 total T cells were infused to yield 1 or 2 x106 BAFF-R-CAR TN/MEM, respectively. Non-transduced T cells from the same donor were used as allogeneic controls (non-CAR). Tumors were monitored by bioluminescent imaging on the days indicated. Both the low and the high dose BAFF-R CAR T cell groups demonstrated complete Nalm-6 CD19KO tumor regressions, compared with controls (Figure, part B).
Conclusions: Our studies support the clinical development of BAFF-R CAR T cells and a pending clinical trial of BAFF-R CAR T cell therapy in relapsed/refractory B-ALL patients who have failed prior CD19-targeted immunotherapy. Targeting BAFF-R may thereby add to existing alternative strategies to overcome relapse from CD19 antigen loss. While BAFF-R antigen loss by tumor cells is unlikely, because its expression is critically required for normal B-cell survival, this hypothesis can only be established by clinical testing.
Qin:InnoLifes: Consultancy, Equity Ownership; Pepromene Bio: Consultancy, Equity Ownership. Aldoss:AUTO1: Consultancy; Helocyte: Consultancy, Honoraria, Other: travel/accommodation/expenses; Jazz Pharmaceuticals: Honoraria, Other: travel/accommodation/expenses, Speakers Bureau; Agios: Consultancy, Honoraria. Kwak:Pepromene Bio: Consultancy, Equity Ownership, Research Funding; InnoLifes: Consultancy, Equity Ownership; Xeme BioPharma, Inc: Consultancy, Equity Ownership; Enzychem LifeSciences: Consultancy; Celltrion, Inc.: Consultancy; Celltrion Healthcare: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal