Background
Acute myeloid leukemia (AML) in elderly patients is a poor prognosis disease due to many factors that include disease biology, high risk of treatment-related mortality, co-morbid illnesses, geriatric syndromes, and psychosocial factors such as cognitive decline and social isolation. Such factors make it difficult to administer intensive chemotherapy regimens needed to achieve a durable response in older patients with AML.
CPX-351, Vyxeos®, is a fixed combination of the antineoplastic drugs cytarabine and daunorubicin, encased together inside the liposome in a 5:1 molar ratio. CPX-351 preferentially targets leukemic cells to a greater degree than non-leukemic cells in the bone marrow, leading to decreased cytotoxicity against normal hematopoietic cells.
Methods
Thirty newly-diagnosed AML patients aged ≥65yrs, without upper age limit restriction, were treated with CPX-351 as a first intensive therapy (Table 1). The primary efficacy endpoint was overall survival (OS) and the primary safety endpoint was 30-day mortality. Secondary efficacy endpoints included response rate and duration of response and assessment of the relationship between cognitive function, quality of life, and treatment outcome. Cognitive function was measured using the Montreal Cognitive Assessment (MOCA) and the Blessed Orientation-Memory-Concentration (BOMC) tests. Quality of life was assessed using the Functional Assessment of Cancer Treatment- Leukemia (FACT-Leu) questionnaire.
Results
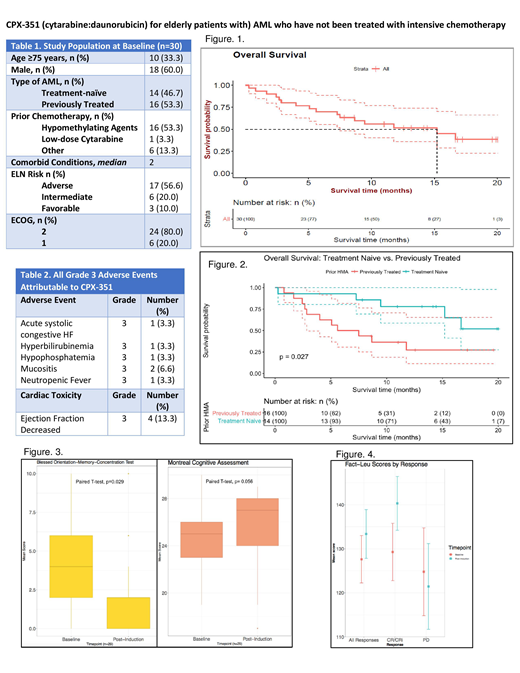
Eleven patients (36.6%) achieved complete remission (CR) and 5 patients (16.6%) achieved complete remission with incomplete platelet recovery (CRi). Of these patients, 8 (26.6%) received stem cell transplants. 2 patients (6.6%) died within 30-days. Of the patients who achieved CR/CRi, 5 (16.6%) relapsed after a median remission duration of 120 days. OS at Day-30 was 93.3% and at Day-60 was 86.6%. Median OS for all patients was 14.5 months (Figure 1). Median event-free survival for all patients (defined as the time from Day 1 of treatment to persistent disease (PD) or death) has not been met at 20 months. Patients who had received prior low intensity therapy (n=16) versus those who were treatment naïve (n=14) had a significantly decreased OS (p= 0.027) (Figure 2). Median OS for previously treated patients was 5.6 months, in contrast to treatment naïve patients, who have not met median OS at 20 months. Patients who had received prior low intensity therapy also had a lower CR+CRi rate of 31.5%, in comparison to treatment naïve patients who had a CR+CRi rate of 78.5%. The most common adverse events attributable to CPX-351 were Grade 1-2 rash (20 patients, 66.7%) and Grade 2-3 decrease in left ventricular ejection fraction (LVEF) (6 patients, 20%) (Table 2). Of patients who experienced a decrease in LVEF, 5 recovered to baseline after a median of 59 days.
There was a significant increase in BOMC score from baseline (BL) to the end of the first induction (EOI) (p=0.029) (Figure 3) for 29 patients. There was also a non-significant increase in the total MOCA score from BL to EOI (p=0.056) (Figure 3) for 29 patients. In addition, there was an expected overall upward trend in total FACT-Leu score from BL to EOI (n=19). There was also an upward trend in total FACT-Leu score from BL to EOI in patients who achieved CR/CRi (n=12). In contrast, there was a downward trend in total FACT-Leu score from BL to EOI in patients with PD (n=7) (Figure 4).
Conclusion:
Elderly AML patients can be treated safely with CPX-351 with a low 30-day mortality, a CR+CRi rate of 53.3%, and a prolonged duration of treatment response with median EFS not met at 20m. Although sample sizes for questionnaire data were small, the observed trends toward improved BOMC, MOCA, and FACT-Leu scores suggest that there may be functional cognitive improvement and improved quality of life for elderly patients treated with CPX-351.
Ritchie:Genentech: Other: Advisory board; Tolero: Other: Advisory board; Pfizer: Other: Advisory board, travel support; agios: Other: Advisory board; Celgene: Other: Advisory board; Jazz Pharmaceuticals: Research Funding; Celgene, Novartis: Other: travel support; AStella, Bristol-Myers Squibb, Novartis, NS Pharma, Pfizer: Research Funding; Ariad, Celgene, Incyte, Novartis: Speakers Bureau; Celgene, Incyte, Novartis, Pfizer: Consultancy. Lee:Helsinn: Consultancy; Jazz Pharmaceuticals, Inc: Consultancy; Roche Molecular Systems: Consultancy; AstraZeneca Pharmaceuticals: Consultancy; Karyopharm Therapeutics: Consultancy; Ai Therapeutics: Research Funding. Desai:Cellerant: Consultancy; Astex: Research Funding; Astellas: Honoraria; Sanofi: Consultancy; Celgene: Consultancy. Roboz:Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Orsenix: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eisai: Consultancy, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amphivena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal