Introduction: AML-MRC is an AML subtype that includes patients (pts) with (1) a history of myelodysplastic syndrome (MDS) or MDS/myeloproliferative neoplasm, (2) a MDS-related cytogenetic abnormality, or (3) multilineage dysplasia in >50% of ≥2 cell lineages in the absence of NPM1 or biallelic CEBPA mutations, per the WHO 2016 AML guidelines. Historically, pts with AML-MRC have poor outcomes with conventional chemotherapy. CPX-351 (Vyxeos®; daunorubicin and cytarabine liposome for injection), a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic 5:1 molar ratio, is approved by the FDA and EMA for the treatment of adults with newly diagnosed, therapy-related AML or AML-MRC. This approval was based on results of a large randomized, open-label, multicenter, phase 3 study (NCT01696084) in pts aged 60-75 years with newly diagnosed, high-risk/secondary AML. In the overall study population, induction followed by consolidation with CPX-351 significantly improved overall survival (OS; 9.56 vs 5.95 months; hazard ratio [HR] = 0.69; 1-sided P = 0.003) vs conventional 7+3, with a safety profile comparable to that of 7+3. An exploratory subgroup analysis of this phase 3 study was performed to compare outcomes for CPX-351 vs 7+3 in pts with AML-MRC who received consolidation.
Methods: Pts were randomized 1:1 to receive 1-2 induction cycles with CPX-351 (100 units/m2 [cytarabine 100 mg/m2 +daunorubicin 44 mg/m2] as a 90-minute infusion on Days 1, 3, and 5 [2nd induction: Days 1 and 3]) or 7+3 (cytarabine 100 mg/m2/day continuously for 7 days [2nd induction: 5 days] + daunorubicin 60 mg/m2 on Days 1-3 [2nd induction: Days 1-2]). Pts achieving a complete remission (CR) or CR with incomplete neutrophil or platelet recovery (CRi) could receive up to 2 consolidation cycles with CPX-351 (65 units/m2 [cytarabine 65 mg/m2 + daunorubicin 29 mg/m2] on Days 1 and 3) or 5+2 (as for 2nd induction). Pts could receive hematopoietic cell transplantation (HCT) at the discretion of the treating physician. This exploratory analysis included the subgroup of pts who met the WHO 2008 AML-MRC criteria and received consolidation.
Results: The study enrolled 309 pts, all with high-risk/secondary AML, including 246 (80%; n = 123 in each arm) who were diagnosed with AML-MRC. Among the pts with AML-MRC, 39/123 (32%) in the CPX-351 arm and 25/123 (20%) in the 7+3/5+2 arm received consolidation and were included in this analysis. Baseline characteristics for this subgroup were generally balanced between arms; however, the CPX-351 arm included a higher proportion of pts with de novo AML with MDS karyotype (46% vs 24%) and a lower proportion of pts with antecedent MDS (44% vs 64%).
The median time from induction last dose to consolidation 1 was 50 days in the CPX-351 arm and 45 days in the 7+3/5+2 arm, and median time between consolidation cycles for those who received 2 cycles was 47 days and 41.5 days, respectively. CPX-351 and 5+2 were received in the outpatient setting by 20/39 (51%) and 1/25 (4%) pts, respectively, during consolidation 1 and 10/17 (59%) and 0/10 pts during consolidation 2.
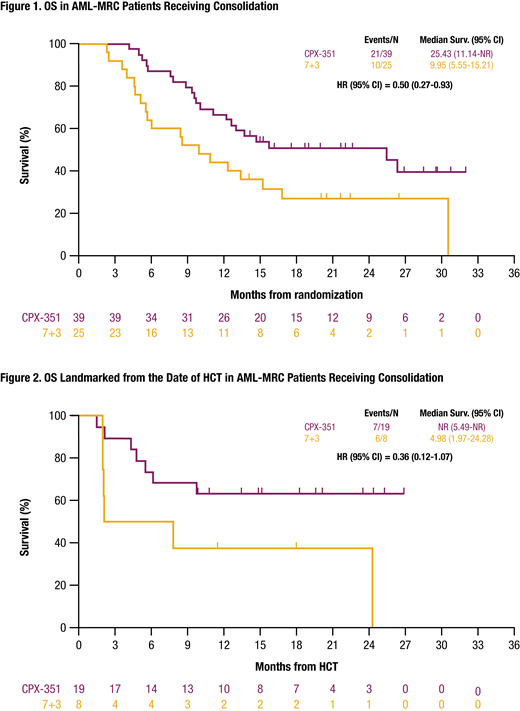
All pts with AML-MRC who received consolidation achieved CR+CRi except 1 patient in the CPX-351 arm. Median OS for pts who received consolidation was longer with CPX-351 vs 7+3/5+2 (25.43 vs 9.95 months; HR = 0.50 [95% CI: 0.27-0.93]; Figure 1). More AML-MRC pts underwent HCT after CPX-351 vs 5+2 consolidation (49% vs 32%; unadjusted relative risk = 1.52 [95% CI: 0.79-2.93]), and OS landmarked from the HCT date was longer with CPX-351 (not reached vs 4.98 months; HR = 0.36 [95% CI: 0.12-1.07]; Figure 2).
The most common treatment-emergent adverse events (TEAEs) in pts with AML-MRC who received consolidation were febrile neutropenia (CPX-351: 82%; 7+3/5+2: 76%), constipation (62%; 64%), peripheral edema (62%; 64%), nausea (62%; 56%), fatigue (51%; 56%), and diarrhea (49%; 80%). The most common grade ≥3 TEAEs were febrile neutropenia (CPX-351: 82%; 7+3/5+2: 76%) and pneumonia (13%; 20%). The most common serious TEAE was febrile neutropenia (13%; 20%). One (3%) pt in the CPX-351 arm and 3 (12%) pts in the 7+3/5+2 arm experienced a TEAE resulting in death.
Conclusions: Continued therapy with CPX-351 throughout induction and consolidation improved median OS, HCT rates, and OS landmarked from the HCT date vs 7+3/5+2 chemotherapy among pts with AML-MRC. The safety profile of CPX-351 in these pts was also consistent with the known safety profile of 7+3/5+2.
Kolitz:Boeringer-Ingelheim: Research Funding; Roche: Research Funding; Astellas: Research Funding. Ryan:AbbVie: Equity Ownership; University of Rochester: Patents & Royalties. Strickland:Sunesis Pharmaceuticals: Research Funding; Pfizer: Consultancy; Kite: Consultancy; Astellas Pharma: Consultancy; Jazz: Consultancy; AbbVie: Consultancy. Schiller:Amgen: Other, Research Funding; Astellas: Research Funding; Biomed Valley Discoveries: Research Funding; Celgene: Research Funding, Speakers Bureau; Bristol Myer Squibb: Research Funding; Agios: Research Funding, Speakers Bureau; Constellation Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Eli Lilly and Company: Research Funding; FujiFilm: Research Funding; Genzyme: Research Funding; Gilead: Research Funding; Incyte: Research Funding; J&J: Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Onconova: Research Funding; Pfizer Pharmaceuticals: Equity Ownership, Research Funding; Sangamo Therapeutics: Research Funding. Ryan:Jazz Pharmaceuticals: Employment, Equity Ownership. Faderl:Jazz Pharmaceutics: Employment, Equity Ownership. Cortes:Astellas Pharma: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal