
Introduction: Patients with acute myeloid leukemia (AML) have a high risk of infection during induction therapy due to the severity and duration of chemotherapy-induced neutropenia. As a result, the American Society of Clinical Oncology and the Infectious Diseases Society of America recommend fluoroquinolone prophylaxis in this patient population during induction therapy. The initial studies supporting the current guidelines showed decreased risk of neutropenic fever and systemic bacterial infections with fluoroquinolone prophylaxis but no mortality benefit. Notably, the use of fluoroquinolone prophylaxis is associated with colonization and infection with multidrug-resistant organisms, and these studies were conducted in areas with low rates of fluoroquinolone resistance. At our institution, the rates of fluoroquinolone resistance among the 3 most common causes of Gram-negative bacteremia (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa) ranged from 17% to 45% among inpatients during the study period. We aimed to evaluate the efficacy of fluoroquinolone prophylaxis in a population with high rates of fluoroquinolone resistance.
Methods: We performed a retrospective chart review of newly diagnosed adult AML patients who received induction therapy at Mount Sinai Hospital in New York, NY, from 6/1/2012 to 12/31/2016. Patients were excluded if they received antibiotics for >4 days in the 1 week prior to induction therapy, developed a clinically or microbiologically documented infection (CDI or MDI) in the 2 weeks prior to induction therapy, did not develop neutropenia (defined as neutrophil count < 500 cells/μL), or developed fever on the first day of neutropenia. Patients were followed for 6 months after initiation of induction therapy. The primary outcome was development of neutropenic fever. The secondary outcomes were development of infections, infection with multidrug-resistant organisms, and mortality. We used a time-varying (Simon-Makuch) statistical approach to categorize patients into the prophylaxis group or no prophylaxis group. We adjusted each outcome for gender, age at diagnosis, type of AML (de novo vs. secondary), and type of induction therapy.
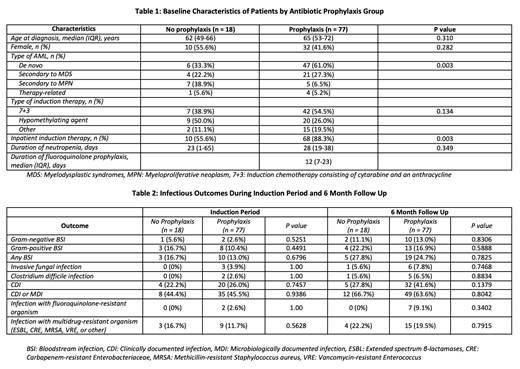
Results: Of the 95 included patients, 77 received primary fluoroquinolone prophylaxis and 18 did not receive any bacterial prophylaxis. There was no difference in median age, gender, or type of induction therapy between the prophylaxis and no prophylaxis groups (Table 1). Although more patients in the prophylaxis group had de novo disease (61% vs. 33%, p=0.003) and received inpatient induction therapy (88% vs. 56%, p=0.003), there was no difference in duration of neutropenia (28 vs. 23 days, p=0.349). There was no statistically significant difference in risk of febrile neutropenia (multivariable HR 1.41 [0.70 - 2.84], p=0.339) or mortality (multivariable HR 0.72 [0.35-1.48], p=0.371), with or without adjustment for baseline confounders. During the induction period and 6-month follow up, there was no difference in rates of bacteremia, invasive fungal infections, Clostridium difficile infections, CDI, MDI, or infection with fluoroquinolone-resistant or multidrug-resistant organisms (Table 2).
Conclusions: Use of primary fluoroquinolone prophylaxis during AML induction therapy did not impact rates of neutropenic fever, infections, and antimicrobial resistance during induction and 6 month follow up. Similar to prior studies, fluoroquinolone prophylaxis also did not improve overall survival. This suggests that primary fluoroquinolone prophylaxis in this high-risk population provides neither benefit nor harm. Larger randomized controlled clinical trials are needed to further investigate this topic.
Jacobs:Ansun Biopharma, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal