KEY WORDS: Acute Promyelocytic Leukemia, oral Arsenic Trioxide, ATRA, Front Line Therapy.
CONTEXT: Studies showed the combination of all-trans-retinoic acid (ATRA) with Arsenic trioxide (ATO) as an effective treatment of patients with newly diagnosed Acute Promyelocytic Leukemia (APL), permitting a chemotherapy-free treatment approach. Oral formulations of ATO were developed with similar efficacy compared to the IV formulation, and a slightly safer profile.
OBJECTIVE:To determine the effectiveness and safety of a locally produced oral ATO with ATRA as a first line therapy in APL.
DESIGN:We designed the LPCR05 protocol based on established treatment protocols. Treatment regimen: Induction phase: all patients received ATRA (45 mg/m2 daily) and an locally produced oral formulation of ATO (10 mg daily), with a dose of Idarubicin (12 mg/m2 on days 1,3,5 and 7, if older than 60 years only given on day 1) added only to the high-risk patients. Consolidation Phase: 3 cycles of ATO + ATRA for 30days every 6 weeks. Maintenance Phase: 4 Cycles of ATRA+ATO daily for 15 days every 3 months. Oral formulation for ATO is prepared by the Laboratory of Pharmaceutical Products from our Social Security System according to the literature and approved by local health and pharmaceutical regulators.
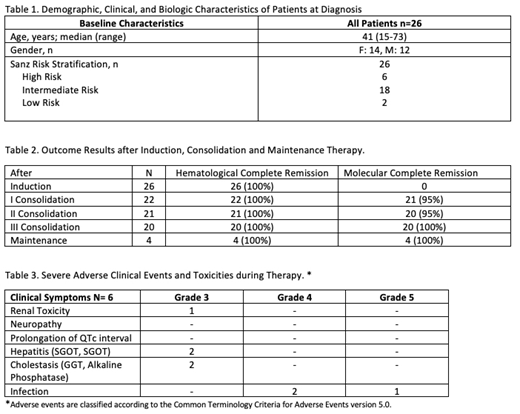
RESULTS: We report data on our first 26 APL patients enrolled on the LPCR05 protocol. Six patients were classified as high risk, eighteen intermediate and two low risk (Table 1). One high risk patient discontinued after first consolidation because of lost follow up. The median follow up is 12.5 months. Hematological complete remission was obtained after 30 days of treatment in all 26 patients. Molecular complete remission in 22 evaluated patients after first and third consolidation was achieved in 95% and 100% respectively (Table 2). None of the patients have relapsed. There were no withdrawals of the protocol because of side effects and no induction deaths because of coagulopathy. No severe cardiac or neurological toxicity was found. Grade 3 hepatic toxicity was seen in two patients, in both cases, temporary discontinuation of ATRA and ATO resolved the side effect. One patient developed non treatment related grade 3 renal toxicity and other one developed a probably related grade 3 skin toxicity. Severe infectious complications were seen in three patients, two had pneumoniae (one death) and one enteritis (Table 3). Patients are managed in the ambulatory setting, with fewer hospitalizations (mean hospitalization days per patient of 38,1 with ATRA + chemotherapy vs 14,1 with oral ATO and ATRA), and therefore the cost is three times lower (US$65 208 vs US$24 195) than our previous approach with ATRA + chemotherapy protocol.
CONCLUSION: Our data demonstrate both efficacy and safety of oral ATO with ATRA as front line therapy in all risk groups. This strategy also demonstrated lower operational costs, fewer hospitalization days, and improved convenience for patients. We expect longer follow-up will confirm that treatment with oral ATO and ATRA is a curative therapeutic strategy for patients with APL that is particularly attractive for use in low and medium income countries.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal