Introduction: R/R FLT3-ITD AML is an aggressive hematologic malignancy with a generally poor prognosis and high relapse rate even after allogeneic hematopoietic stem cell transplantation (HSCT). QuANTUM-R (NCT02039726), a large, global, phase 3, randomized, controlled trial, showed that single-agent quizartinib significantly prolonged overall survival (OS) vs salvage chemotherapy (SC) in patients with R/R FLT3-ITD AML after first-line treatment with or without HSCT (Cortes et al, Lancet Oncol 2019). To evaluate both quality and quantity of life for patients with R/R FLT3-ITD AML, we conducted a Q-TWiST analysis of QuANTUM-R data to compare survival between patients receiving quizartinib and SC adjusted for quality of life (QOL).
Methods: The primary analysis cohort was the intent-to-treat (ITT) QuANTUM-R population, which included 367 patients (245 patients receiving quizartinib; 122 patients receiving SC). The secondary analysis cohort was the per-protocol analysis set (PPS), which excluded patients randomized but not treated and patients with major protocol violations who would affect assessment of efficacy endpoints (231 patients receiving quizartinib; 88 patients receiving SC).
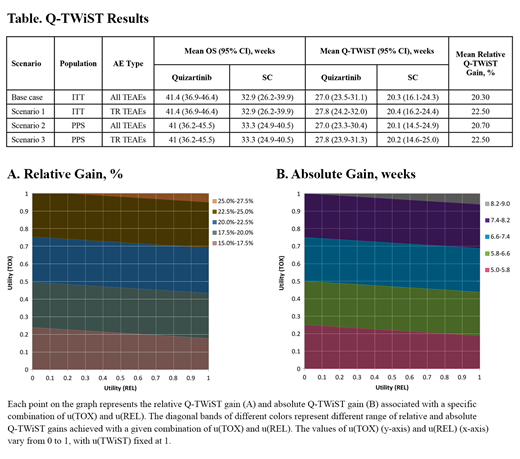
Each patient's OS was partitioned into 3 health states: time with any grade 3/4 treatment-emergent adverse events (TEAEs) before relapse (TOX), time without relapse or grade 3/4 toxicity (TWiST), and time after relapse (REL). Q-TWiST was assessed at 104 weeks (approximate median follow-up of QuANTUM-R) as the mean time spent in each state weighted by its respective QOL, represented by health utility (u; 0.0-1.0). Q-TWiST was calculated as follows:
Q-TWiST = u(TWiST) × TWiST + u(TOX) × TOX + u(REL) × REL
for which u(TWiST), u(TOX), and u(REL) represent the utilities applied to the restricted mean time in TWiST, TOX, and REL, respectively.
Relative Q-TWiST gain (ie, Q-TWIST gain divided by mean OS of salvage chemotherapy) of ≥ 15% was considered clinically important, based on published minimally important difference norms (Revicki et al, Qual Life Res 2006).
The base case that assessed the Q-TWiST difference between quizartinib and SC for the ITT population included any grade 3/4 TEAEs and set utilities at commonly assumed values: u(REL) = u(TOX) = 0.5, relative to u(TWiST) = 1.0. A sensitivity analysis was conducted for which u(TOX) and u(REL) were varied from 0.0 to 1.0. The following additional scenarios analyses were conducted: (1) including only treatment-related (TR) grade 3/4 TEAEs, (2) assessing Q-TWiST difference between quizartinib and SC for the PPS cohort, and (3) including only TR grade 3/4 TEAEs for the PPS cohort. 95% CIs were estimated via nonparametric bootstrap.
Results: For the ITT population, quizartinib was associated with a mean OS gain of 8.5 weeks vs SC at 104 weeks from randomization (Table). In base case, the mean (95% CI) Q-TWiST gain at 104 weeks for quizartinib vs SC was 6.7 weeks (1.7-12.3; relative gain, 20.3%), which was statistically significant and clinically meaningful (Table). For the sensitivity analysis for which u(TOX) and u(REL) were varied from 0.0 to 1.0, Q-TWiST gains for quizartinib ranged from 5.0 (u[REL] = u[TOX] = 0.0) to 8.5 weeks (u[REL] = u[TOX] = 1.0). The relative Q-TWiST gains for quizartinib vs SC remained clinically important, irrespective of the values for u(TOX) and u(REL) and ranged from 15.2% to 25.5% (Figure). In the scenario analyses (Table), (1) when only TR grade 3/4 TEAEs were included, the Q-TWiST gain for quizartinib was 7.4 weeks (2.2-13.0; relative gain, 22.5%), (2) in the PPS cohort, the mean (95 % CI) Q-TWiST gain for quizartinib was 6.9 weeks (0.8-13.5; relative gain, 20.7%), and (3) when including only TR grade 3/4 TEAEs in the PPS, the Q-TWiST gain for quizartinib was 7.5 weeks (1.5-14.2; relative gain, 22.5%).
Conclusions: Quizartinib significantly prolonged quality-adjusted survival vs SC in patients with R/R FLT3-ITD AML after disease control, safety, and QOL were accounted for, providing evidence of meaningful clinical benefit in a patient population with few treatment options. To put these results in perspective, the relative Q-TWiST gains for quizartinib reported herein are larger than nearly 90% of the relative Q-TWiST gains reported in a recent systematic review of 81 Q-TWiST comparisons across 13 cancers (Solem et al, Exp Rev Pharmacoecon Outcomes Res 2018).
Cortes:Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; BiolineRx: Consultancy; Sun Pharma: Research Funding. Ganguly:Daiichi Sankyo: Research Funding; Kite Pharma: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Seattle Genetics: Speakers Bureau. Khaled:Omeros: Consultancy; Alexion: Consultancy, Speakers Bureau; Daiichi Sankyo: Other: Travel support. Krämer:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; BMS: Research Funding. Levis:Daiichi Sankyo Inc: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; FUJIFILM: Consultancy, Research Funding. Martinelli:Janssen: Consultancy, Other: trial grant; Celgene: Consultancy, Honoraria, Other: trial grant; Abbvie: Consultancy, Honoraria, Other: trial grant; Pfizer: Consultancy, Other: trial grant; Roche: Consultancy, Other: trial grant; Incyte: Consultancy, Other: trial grant; Daiichi Sankyo: Consultancy, Honoraria; Amgen: Consultancy, Other: trial grant; Novartis: Consultancy, Other: trial grant; Ariad: Consultancy, Other: trial grant. Perl:Bayer: Research Funding; BioMed Valley Discoveries: Research Funding; FujiFilm: Research Funding; Novartis: Honoraria, Other: Advisory board, Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of the data., Research Funding; Jazz: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; AbbVie: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Actinium Pharmaceuticals: Consultancy, Honoraria, Other: Clinical Advisory Board member, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Non-financial support included travel costs for advisory board meetings.; Daiichi Sankyo: Consultancy, Honoraria, Other, Research Funding; Arog: Consultancy, Other: Non-financial support included travel costs for advisory board meetings.; Astellas: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of trial data., Research Funding; NewLink Genetics: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Takeda: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.. Russell:Astellas: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau; DSI: Consultancy, Honoraria, Speakers Bureau; Pfizer Inc: Consultancy, Honoraria, Speakers Bureau. Botteman:Pharmerit International: Employment, Equity Ownership; Alnylam Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy, Research Funding. Shah:Merck: Research Funding; Bayer: Research Funding; Pfizer: Research Funding. Luo:Pharmerit International: Employment. Shun:Daiichi Sankyo: Employment. Ray:Daiichi Sankyo: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal