Infants with acute leukemia present special challenges, due in part to the unusual features of their disease. A clonal oncofusion protein is detectable in the vast majority of infants presenting with acute leukemia, whether myeloid (AML), lymphoblastic (ALL), or mixed-phenotype (MPAL). Many recurrent oncofusion proteins seen in this age group involve the KMT2A (formerly MLL, Mixed Lineage Leukemia) gene, located on chromosomal band 11q23, which is notorious for both lineage switching and poor clinical prognosis. Other fusions involve components of the nuclear pore complex (NUP98), chromatin modifiers (KAT6A), or lineage-associated pioneer factors (such as MYB and GLIS2). This diversity of oncofusion proteins has hampered efforts to develop targeted agents, and over half of infant cases eventually succumb to their disease. Previous work showed that infant ALL is distinct from childhood or adult ALL. Here we show that infant AML is similarly distinct from AML in other age groups, and this difference yields transformative opportunities to repurpose existing targeted and immune therapies.
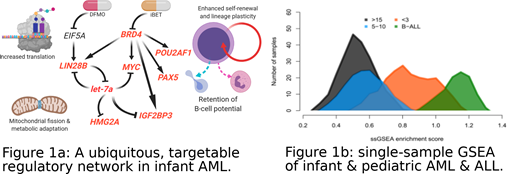
mRNA sequencing (RNAseq) analysis of over 1500 participants in Children's Oncology Group clinical trials revealed hundreds of infant-AML-specific transcripts, many associated with early B cell development. 18 of the affected genes form a canonical B-cell regulatory circuit, including the epigenetic regulators BRD4 and POU2AF1, and their onco-fetal targets LIN28B and IGF2BP3. These four genes also host hypo-methylated super-enhancers in most infant AML cases, but in few older patients. The MYC proto-oncogene is directly implicated in a regulatory loop involving microRNA let7a-2, which is expressed in a mutually exclusive manner with its target and regulator LIN28B (Fig 1A). By contrast, the WT1 gene, abundantly expressed in over 90% of AML cases from other age groups, is transcribed at low levels in most infant AML cases. Single-sample gene set enrichment analysis revealed that most cases of infant AML bear more similarity to infant B-cell ALL or MPAL than to AML in older children, adolescents, and adults (Fig 1B). Higher-order chromatin conformation further emphasizes these differences.
The MYC proto-oncogene, a critical node in the gene regulatory network (Fig. 1A) observed across infant acute leukemia cases, is routinely amplified via trisomy 8 in this age group (and in fact all childhood leukemia). The IGF2BP3 gene, another critical node (Fig. 1A), is rarely deleted in infants (deletions of 7q tend to spare 7p15, and complex karyotype cases often retain two or more copies on derivative chromosomes). The immature humoral and adaptive immune system in infants complicates treatment in this age group, but also yields metabolic vulnerabilities which can be exploited to break the nearly ubiquitous positive feedback loop described herein.
These age-specific differences suggest that alternative therapeutic targets and modalities are warranted not only in infant AML, but across infant acute leukemia more generally. The relatively small numbers of infant acute leukemia cases by lineage, along with common mechanisms that appear to sustain most cases of infant acute leukemia, and frequent clinical reports of lineage switching in infants regardless of morphology at presentation, all support this contention. Our results indicate that combination therapies coupling experimental ages (such as DFMO, which targets metabolic regulation of MYC, LIN28B, and let-7a via ornithine decarboxylase, and has shown substantial efficacy in MYCN-amplified neuroblastoma) with already approved agents such as BET inhibitors (which target BRD4) and blinatumomab (targeting CD19 as a canonical B-cell antigen) may be warranted. These immune-targeting combinations represent promising and potentially lineage-agnostic approach to improving outcomes for infants with acute leukemia, a group of patients for whom novel therapeutic strategies are desperately needed, and for whom less toxic, more effective, targeted combination therapies may spare a lifetime of late effects.
Pardo:Hematologics, Inc: Employment. Kaeding:Celgene: Employment. Loken:Hematologics, Inc: Employment, Equity Ownership. Farrar:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal