Background
Allogeneic hematopoietic stem cell transplantation (alloHSCT) has been established as a powerful treatment modality for patients with hematological malignancies. The graft-versus-leukemia effect, however, is strongly associated with the occurrence of graft-versus-host disease (GVHD) and subsequent transplant-related mortality. The current standard Dutch regimen for prevention of GVHD in T-cell replete (TCR) alloHSCT following reduced intensity conditioning (RIC) consists of mycophenolic acid (MA) and cyclosporine A (CyA) for three and six months post-transplant, respectively. However, as approximately 30% of patients will never develop GVHD, immunosuppressive overtreatment is of concern and might impair outcome.
Aims
In the present prospective randomized, multicenter, phase III trial we set out to compare time-restricted immunosuppression versus a standard immunosuppressive regimen. The primary objective was to increase the proportion of patients with non-severe GVHD (either acute GVHD grades I-II without gut involvement or chronic GVHD not requiring systemic treatment) within 180 days after transplantation and to reduce the relapse rate, without increasing severe GVHD. Secondary endpoints included time to acute and chronic GVHD, progression-free survival (PFS), GVHD-free/relapse-free survival (GRFS), overall survival (OS), and adverse events.
Methods
Hematological patients planned to undergo TCR alloHSCT with a related or unrelated 8/8 HLA matched donor were included. The trial randomized patients between three treatment arms. The current analysis includes all patients randomized between arms A and B. Immunosuppression consisted of CyA twice daily aiming for serum trough levels of 250-350 µg/L and MA 16 mg/kg twice daily with a maximum dose of 2160 mg a day. The standard regimen (arm A) prescribed to discontinue MA at day 84 post-transplant and CyA was continued until day +120 followed by tapering until day +180. In those randomized for the time-restricted regimen (arm B) MA was discontinued at day 28 post-transplant and CyA was continued until day +84 followed by tapering.
Results
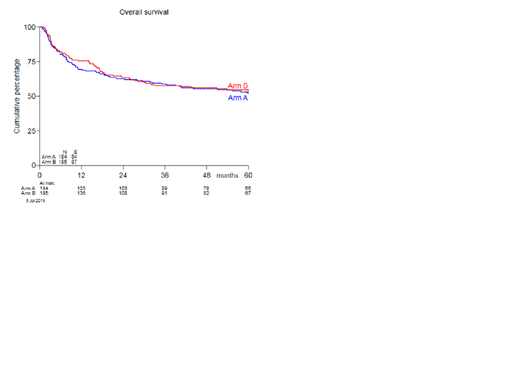
A total of 389 patients were randomized 1:1 between arms A and B, of whom 95% (184 in arm A versus 185 in arm B) proceeded to transplant. The median age was 55 (range: 18-71), 57% were male. Fifty-one patients received myeloablative conditioning and 318 (86%) patients RIC. Donors were matched siblings for 135 patients and matched unrelated donor (MUD) for 233 patients. The majority of patients received peripheral blood stem cells, consisting of median 6.46x106/kg CD34+ cells/kg (range: 0.94-26.3) and median 230x106/kg CD3+ T cells (range: 0-936). Baseline patient and transplantation characteristics were equally distributed between the treatment arms. The proportion of patients developing non-severe GVHD within 180 days post-alloHSCT was 24% in both treatment arms, with an odds ratio (OR) of 1.01 (95% confidence interval 0.61-1.67, p 0.98). The cumulative incidence (CI) of grade II-IV and grade III-IV acute GVHD at 6 months post-alloHSCT was not significantly different between the two arms (47% and 15% versus 52% and 18%), nor was the maximum grade or organ involvement of acute GVHD. In addition, no difference was seen in the two-year CI of chronic extensive GVHD between the two treatment arms (51% versus 49%). The three-year estimate of PFS was 51% (44-59%) versus 52% (44-59%), respectively. The three-year CI of progression/relapse was 28% in arm A versus 27% in arm B. OS at three years was 59% (0.51-0.66%) versus 57% (0.50-0.64%). The one-year estimate of the composite endpoint GRFS was 14% (9-19%) in arm A, and 17% (12-22%) in arm B. Incidences and nature of adverse events were comparable in both arms.
Conclusion
A time-restricted combination of MA and CyA did not increase the proportion of patients with non-severe GVHD within 180 days after transplantation and resulted in similar outcome as compared to standard immunosuppression following alloHSCT using sibling and well-matched unrelated donors. Given the observed high CI of acute grade II-IV and chronic extensive GVHD and low GRFS for both the time-restricted and standard regimen, recipients of TCR RIC alloHSCT should be considered for more intensive immunosuppression.
Nur:Novartis: Consultancy. Deeren:Alexion, Amgen, Janssen, Roche, Sunesis, Takeda, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal