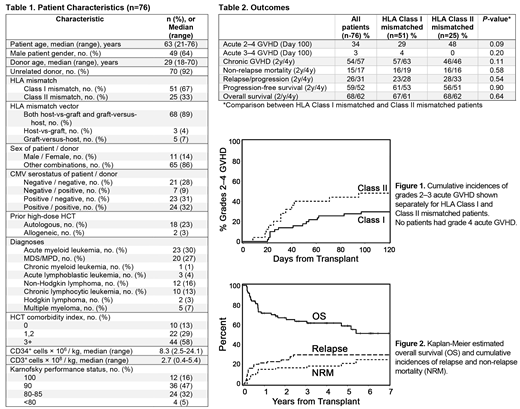
The success of HCT is highly dependent on the availability of compatible donors. Depending on ethnicity, fully HLA-matched donors cannot be identified for 25-84% of patients. In an effort to extend the use of HLA-mismatched donors to patients only eligible for NMA HCT, we previously demonstrated that engraftment and long-term survival can be achieved using CSP and MMF immunosuppression after HLA class I antigen-mismatched HCT (Nakamae et al, 2010). Based on this trial, we designed a phase II multi-center trial to assess the efficacy of GVHD prophylaxis with triple immune suppression with CSP, MMF and sirolimus after HLA class I or class II antigen mismatched NMA HCT (fludarabine 90mg/m2 and 2-3 Gy TBI). Patients ineligible for high-dose conditioning with only HLA class I or class II antigen mismatched donors available were eligible for inclusion. The primary objective was to determine whether the incidence of grades 2-4 acute GVHD can be reduced to less than the historical rate of 70% with the triple-immunosuppression. The evaluation was planned to be carried out separately among HLA class I and class II mismatched patients. Secondary objectives included day 100 non-relapse mortality (NRM) and grades 3-4 acute GVHD. CSP was started on day -3 and continued to day +150 and then tapered off by day +180. Sirolimus was started on day -3 through day +180 and then tapered off by day +365. MMF was given thrice daily from days 0 to +30 then twice daily to day +100 and tapered off by day +150. A total of 76 patients were enrolled and received conditioning with fludarabine and TBI followed by infusion of unmodified G-CSF mobilized peripheral blood stem cell (PBSC) grafts. The median age was 63 (21-76) years, and median follow-up of surviving patients was 47 (4-94) months. 51 (67%) and 25 (33%) of patients had HLA class I or II mismatches, respectively. Transplant demographics are summarized in Table 1.
Sustained engraftment was observed in all but 1 (1%) patient with myeloproliferative disease who had autologous recovery after transplant. Median number of days with neutrophils < 500 cells/ml and platelets <20,000 platelets/ml were 14 (0-67) and 2 (0-70), respectively. Full donor T-cell chimerism (>95% donor) was achieved in 48% and 63% at days +28 and 84, respectively.
Outcomes are summarized in Table 2.The cumulative incidence of grade 2-4 acute GVHD at day +100 was 34% in all patients (29% and 48% after HLA class I and class II mismatched, respectively; p=0.09) (Figure 1), with only 3% of patients experiencing grade 3 acute GVHD and no patients experienced grade 4 acute GVHD.
NRM at day +100, 2 years and 4 years were 4%, 15% and 17%, respectively (HLA class I vs class II: p=0.58) (Figure 2). The 2- and 4-year cumulative incidences of chronic GVHD were 54% and 57% (HLA class I vs. class II: p=0.11). Relapse incidences at 2 and 4 years were 26% and 31% with no differences between the class I and II mismatched patients (p=0.54) (Figure 2). The cumulative incidences at 2 and 4 years of progression-free survival (PFS) were 59% and 52%, and overall survival (OS) were 68% and 62% with no differences between HLA class I and II mismatches for PFS or OS (p=0.64 and 0.90, respectively) (Figure 2).
Compared to historical data from our previous trial using MMF and CSP prophylaxis alone in patients with HLA class I mismatches, the addition of sirolimus to GVHD prophylaxis with CSP/MMF improved outcomes both after HLA class I or class II mismatched HCT (Nakamae et al, 2010). In a comparison of HLA class I mismatches only, day +100 grade 2-4 acute GVHD was reduced from 69% in the historical cohort to 29% in the current trial. The relapse incidences were comparable, while 2-year NRM decreased from 47%, in historical controls, to 16%, and translated into improved OS from 26% to 67% at 2 years in the current study. No difference was observed in the incidence of chronic GVHD. Furthermore, in the current trial, similar results were seen using HLA class II mismatched donors, suggesting that the triple drug immunosuppression could be used with either type of mismatch.
The current trial demonstrates that triple drug immunosuppression with CSP, MMF, and sirolimus is safe and effective in reducing both acute GVHD and NRM after NMA HCT with either HLA class I or class II antigen mismatched donors.
Maloney:Juno Therapeutics: Honoraria, Patents & Royalties: patients pending , Research Funding; Celgene,Kite Pharma: Honoraria, Research Funding; BioLine RX, Gilead,Genentech,Novartis: Honoraria; A2 Biotherapeutics: Honoraria, Other: Stock options .
In the current study cyclosporine, mycophenolate mofetil and sirolimus are used to prevent graft versus host disease after allogeneic hematopoietic cell transplantation. Fludarabine is used together with total body irradiation as at part of the conditioning prior to allogeneic hematopoietic cell transplantation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal