Introduction: The process of thrombosis depends on a complex interaction between different factors including vascular endothelial activation, platelet activation and adhesion. Blood coagulation process initiated by plasma protease factor VIIa in complex with the membrane protein tissue factor. Cancer-associated thrombosis (CAT) accounts for about 20% of all cases of Venous Thromboembolism (VTE). Tissue factor (TF) is documented to be highly expressed on the cancer cells and pathological angiogenic endothelial cells. Subsequent theories proposed that the cancer cells activate the coagulation cascade, as the oncogenes cause tumor transformation, which in turn causes the cancer tissues to be able to express different pro-coagulant proteins. VTE risk factors can be divided into cancer- and therapy-associated factors.
Low molecular weight heparin (LMWH) is used for prophylaxis and treatment of deep vein thrombosis and CAT as it significantly shortened both duration of thromboembolic crisis and hospitalization. Safety concerns associated with the bleeding risks are the major barrier to dose optimization of treatments. Here, we used the novel sulfated non-anticoagulant LMWH, referred to as S-NACH, which has a similar activity of LMWH but devoid of systemic anti-factor Xa and IIa activity. The sulfated form has enhanced S-NACH binding to the vascular endothelial cells, release TFPI and potentiate the action of tissue factor pathway inhibitors (TFPI). We investigated the effects of S-NACH on clot kinetics in comparison with LMWH using thrombelstography (TEG) and examined its in vitro and in vivo effects on vascular endothelial release capacity of TFPI. Also we investigate the effects of S-NACH on cancer-associated thrombosis-mediated by human pancreatic cancer cells (SUIT2).
Methods: We conducted both in vitro and in vivo experiments using our compound (S-NACH) to test its efficacy on coagulation properties in comparison to LMWH (enoxaparin). We compared the effects on the coagulation time, time to clot initiation and thrombus strength level in vitro, with and without HUVEC endothelial cell line coated into cups used for the TEG analysis. TFPI levels were also estimated in both in vitro and in vivo models at different doses of S-NACH. In addition, the effect of S-NACH on factors Xa and IIa was assessed in mice and rabbits. Also, weused pancreatic cancer cell line (SUIT2) to test the efficiency of blood coagulation in the presence of S-NACH, and Enoxaparin at 1-10 μg.
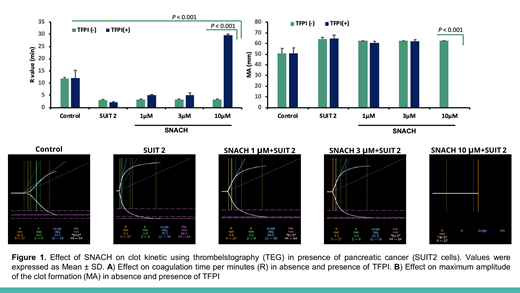
Results: TEG assay have shown that, increasing concentrations of S-NACH (0.1 - 10 ug) did not affect the clotting time (R) and maximum amplitude of the clot formation (MA), while LMWH (enoxaparin) was associated with marked increased clotting time (R) and decreased maximum amplitude of the clot formation (MA) in comparison to control. Using HUVEC cell line coated cups; both S-NACH and LMWH were associated with increased clotting time (R) and decreased maximum amplitude of the clot formation (MA). This is due to the ability of HUVEC cell to release of TFPI. In the in vivo study, administration of enoxaparin inhibited both factors Xa and IIa, while administration of S-NACH did not affect the activities of these coagulation factors. S-NACH caused 3 folds greater tissue factor pathway inhibitor (TFPI) release from human endothelial cells and in mice versus enoxaparin. On the other hand, TEG assay shows significantly decreased clotting time (R-value) and increased clot strength in blood from healthy donors when adding pancreatic SUIT2 cells. Data from the TEG assay demonstrated lack of effects of S-NACH or TFPI alone on clot kinetics in human. In contrast, S-NACH (1-10 μg) in the presence of 0.01-0.1 ng TFPI resulted in a dose-dependent suppression of clot strength and prolongation of clotting time (Figure 1).
Conclusion: S-NACH is devoid of anti-thrombin binding and inhibition of systemic anti-thrombin-dependent coagulation factors such as factor Xa and factor IIa but has an optimal releasing capacity of vascular endothelial TFPI that is associated with antithrombotic activities without any effect on hemostasis. Also, S-NACH prevents CAT without any effects on hemostasis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal