Background: Superficial vein thrombosis (SVT) has been historically considered to have a low risk of distal embolization and mortality, typically not warranting anticoagulation in the absence of underlying risk factors for thrombosis. However, recent studies of outcomes of SVT in patients without malignancy revealed significantly increased incidence of deep venous involvement at the time of presentation, as well as higher than expected rates of complications, reported in up to 10% of patients (Decousus et al. Ann Int Med(152)2010:218-224). However, outcomes of SVT in patients with cancer have not been well studied, despite the known increased risk of malignancy-associated thrombosis. In this study, we examined the rate of complications in patients who developed SVT in the setting of active malignancy.
Methods: A retrospective single-center chart review of electronic medical records along with a corresponding database of radiology reports from NYU Winthrop Hospital from 2013 to 2019 was performed to identify patients with cancer who also had image-confirmed SVT. Patients were included if they had a synchronous or subsequent deep vein thrombosis (DVT) or pulmonary embolism (PE) during treatment, but excluded if they had a history of thrombosis prior to their cancer diagnosis, primary hypercoagulable disorder, or on therapeutic anticoagulation at the time of SVT diagnosis. Descriptive statistics for the overall sample, as well as a subgroup of subjects who were not treated at SVT diagnosis, were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
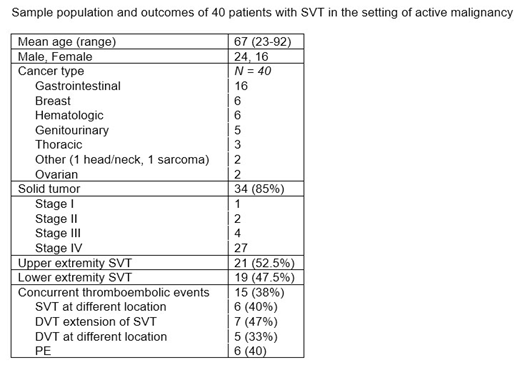
Results: 490 patients were noted to have a diagnosis of SVT and cancer, and of these, 40 met the enrollment criteria. Demographic data are shown in the table below. Among the 40, 21 patients had upper extremity, and 19 had lower extremity SVT. As shown in the table, 15 patients (37.5%) had a concurrent thromboembolism at the time of SVT diagnosis. An intervention was provided in 26 (69% full dose and 11% prophylactic dose anticoagulation, 15.3% antiplatelet therapy, and 3.8% vena cava filter placement). Of these 26 patients, one who received prophylactic anticoagulation developed a subsequent DVT. This patient had stage IV colon cancer and recent surgical resection, and developed SVT associated with intravenous access. Among the 14 patients with SVT who were not treated, 6 (43%) developed subsequent complications including one with DVT at the same extremity, 2 with DVT at a different location, and 3 with recurrence of the underlying SVT. Five of these were stage IV gastrointestinal cancers (including 3 patients with pancreatic cancer, 1 with gastric, and 1 with cholangiocarcinoma), and one had stage IV ovarian cancer. Considering the 8 patients who were not treated and did not develop complications, there was no dominant cancer type (3 were stage IV breast cancer, 1 had stage I pancreatic cancer, 1 had stage III hepatocellular carcinoma, and one had chronic lymphocytic leukemia).
Conclusions: This study reveals a greater degree of concurrent SVT complications in the setting of malignancy compared to prior reports in patients without cancer. The rate of complications were higher in those patients who did not receive treatment (either prophylactic or therapeutic) for their SVT or who had metastatic disease. Although the overall incidence of SVT in cancer patients is low, data presented here suggest a higher than expected rate of complications within a 3 month span following SVT diagnosis in the setting of malignancy compared to prior reports of patients without malignancy. Prospective studies are needed to assess the benefit of anticoagulation in decreasing the risk for SVT complications in patient with active cancer.
Braunstein:Celgene: Consultancy, Other: Advisory boards; Takeda: Consultancy, Other: Advisory Board; Amgen: Consultancy, Other: Advisory Board; AstraZeneca: Consultancy, Other: Advisory Board; Janssen: Consultancy, Other: Advisory Board, Research Funding; Verastem: Consultancy, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal