Normal hemostasis is desired prior to invasive procedures such as surgery. For emergent surgeries, where waiting for an anticoagulant to wear off is not an option, reversal agents or antidotes are often administered. However, for cardiac surgery requiring cardiopulmonary bypass (CPB), hemostatic needs vary throughout the procedure. Early in the procedure, normal hemostasis is preferred for skin incision, sternotomy, and cannulation. However, during CPB, strong anticoagulation is needed, typically with unfractionated heparin (UFH), to prevent thrombosis in the circuit. Thus, any anticoagulant reversal agent administered prior to cardiopulmonary bypass must not interfere with heparinization.
Andexanet alfa (AnXa) is the only FDA-approved reversal agent for oral direct factor Xa (FXa) inhibitors. AnXa is a recombinant FXa variant that lacks the membrane-binding Gla domain and is catalytically inactive due to mutation of the active site serine 195 residue to alanine. It retains active site conformation, and therefore binds active site inhibitors like rivaroxaban, apixaban, and edoxaban, with high affinity. When administered in stoichiometric quantities, it can rapidly sequester direct factor Xa inhibitors and thereby neutralize their effects. However, AnXa has also been reported to reverse the anticoagulant effects of indirect FXa inhibitors, including low molecular weight heparin, fondaparinux, and UFH, both in vitro and in vivo.
Given its biochemical properties and mechanism of action, we hypothesized that administration of AnXa would severely limit the anticoagulant efficacy of UFH and delay safe initiation of CPB. To evaluate this, we measured activated clotting times (ACT) in whole blood from healthy volunteers (N=8) supplemented with varying concentrations of AnXa, UFH, and rivaroxaban. As expected, 4 U/mL UFH, a frequently cited therapeutic concentration for CPB, prolonged the ACT to 418±45s, compared to a baseline ACT of 117±8s. For reference, an ACT of 400s is typically considered the minimum safe level of anticoagulation needed for CPB. In comparison, "low dose" AnXa, 2 mM, in the presence of 4 U/mL UFH and typical blood concentrations of rivaroxaban, 500 nM, resulted in an average ACT of 189±26s, well below the level needed for CPB. In an attempt to overcome the effects of AnXa, we repeated these experiments with supratherapeutic levels of UFH, 16 U/mL, in the presence of 2mM AnXa and rivaroxaban, which prolonged the ACT to 376±36s, but was not reliably above the 400 s threshold. In the absence of AnXa, 16 U/mL heparin resulted in an ACT near 1000 s.
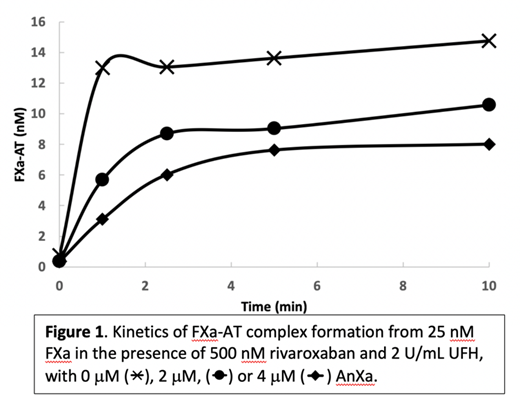
In patients who have recently taken rivaroxaban, a "high dose" of AnXa, approximately 4 mM, is typically used. 4 mM AnXa in the presence of 4 U/mL or 16 U/mL UFH and 500 nM rivaroxaban yielded ACT values of 139±17s and 184±27s, respectively. Administration of recombinant antithrombin III (AT) was not able to overcome the effects of AnXa. To help elucidate the mechanism by which AnXa hinders heparinization, we measured the kinetics of FXa inhibition by AT in the presence of heparin. As shown in Figure 1, AnXa slows the rate of FXa-AT complex formation in a dose-dependent manner. When the stoichiometry of the reaction is taken into consideration, this indicates that AnXa sequesters heparin in a ternary complex with AT which thereby prevents heparin from being free to potentiate AT's interaction with endogenous FXa.
Together, these data suggest that therapeutic heparinization after administration of AnXa is, at best, challenging, if not impossible. From a clinical perspective, this information is crucial in emergent cases as providers will either need to choose alternative anticoagulation strategies for CPB (such as argatroban, bivalirudin, or an emerging FXIa or FXIIa inhibitor), or avoid AnXa and tolerate increased bleeding upon sternotomy prior to CPB. Further, it poses an important safety concern as patients requiring emergent cardiac surgery are often hemodynamically unstable and delays in initiation of CPB due to inability to therapeutically heparinize following AnXa administration could adversely affect outcomes. We are currently evaluating whether the effects of AnXa on heparinization translate to increased thrombosis on CPB oxygenator membranes using an in vitro extracorporeal circulation model.
Patel:Edward's Life Sciences: Other: Speaker. Camire:Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal