Introduction: Replacement factor VIII (FVIII) products have been administered to achieve desired FVIII levels for bleed treatment, perioperative management, or routine prophylaxis. Individual variability in pharmacokinetics (PK) has resulted in dosing ranges, rather than fixed doses. N8-GP (ESPEROCT®) is an extended half-life recombinant FVIII product. The pathfinder trials evaluated routine prophylaxis and bleed treatment in previously treated adolescents/adults (pathfinder 2) and children (pathfinder 5) with severe hemophilia A and perioperative management of major surgery (pathfinder 3).
Methods: Adolescents/adults (aged ≥12 y) were administered 50 IU/kg N8-GP every 4 days (Q4D) as routine prophylaxis in the main phase; alternative weekly dosing was explored in 2 extension phases. For the on-demand treatment arm, patients were to be given 20 to 70 IU/kg, depending on bleed severity and desired FVIII levels. Children (aged <12 y) on prophylaxis were administered a target of 60 (50-75) IU/kg N8-GP twice weekly. For surgery, a pre-trial test dose of 50 IU/kg was administered with PK assessment and then dosing in the study was to achieve desired FVIII suggested by World Federation of Hemophilia guidelines.
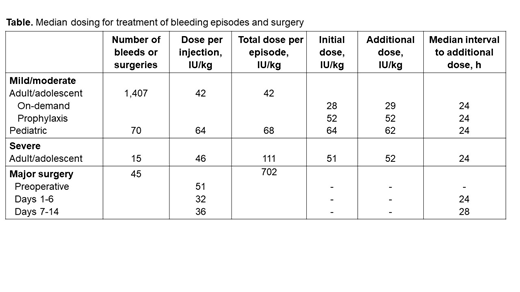
Results: Pathfinder 2 enrolled 186 patients (46 from the US) with 128 (69%) completing the second extension phase encompassing 785 patient-years (66,577 exposure days) with 2,758 treated bleeds. On-demand patients (n=12) treated for a total of 37 patient-years of exposure reported 1,270 (46%) bleeds. In the main phase, 105 of 175 adolescents/adults on prophylaxis (50 IU/kg Q4D) experienced 436 bleeds with a median annualized bleeding rate (ABR) of 1.2. Through the study (mean 3.5 years), 177 adolescents/adults on doses of ~52 IU/kg Q4D had a median ABR of 0.99 and mean (95% CI) FVIII trough levels of 3.1 (2.6-3.4) IU/dL. Treatment of bleeding through extension 1 is described in Table; the median dose for mild/moderate bleeds was 42 IU/kg. For participants who were in the on-demand arm, the median initial dose was 28 IU/kg, with 88.4% of bleeds treated with a single dose. In subjects receiving prophylaxis, the median initial dose matched the prophylaxis dose (52 IU/kg), and 76% of bleeds received a single dose. For 15 severe bleeds, the median total dose was 111 IU/kg per episode.
Pathfinder 5 enrolled 68 children (34 aged 0-5 y, 34 aged 6-11 y), 95% previously on prophylaxis; 62 completed the extension, amounting to 306 patient-years (32,138 exposure days) with mean exposure of 4.5 years. Overall, 55 patients (81%) reported 330 bleeds; most were traumatic (67%). Median ABR in the main phase (0.48 years) was 2.0 and through the entire study was 0.8 with mean (95% CI) FVIII trough activity of 1.9 (1.6-2.5) IU/dL. The mean prophylaxis dose was 64.7 IU/kg at a mean interval of 3.5 days, likely reflecting rounding the targeted 60 IU/kg twice weekly dose. For 70 bleeds in the main phase, 88% of bleeds were treated with 1 to 2 injections; median utilization for bleeds was 68 IU/kg (Table).
Pathfinder 3 evaluated 45 surgeries in 33 adolescents/adults; 96% were reported as excellent/good efficacy. Median preoperative dose mirrored test dose (52 IU/kg) with median total dose per surgery of 702 IU/kg (until 14 days post-surgery). Postoperative dosing was at ~24-hour intervals with the number of doses and treatment duration dependent on procedure (Table).
Conclusion: The pathfinder trials demonstrate effective prophylaxis was achieved with very consistent prophylaxis dosing for both adolescents/adults (~50 IU/kg Q4D) and children (~65 IU/kg twice weekly). Bleeds were generally treated for patients on prophylaxis with a single prophylactic dose (~50 or ~65 IU/kg). Dosing intervals in severe bleeding and surgery were ~24 hours, including in children.
Escobar:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; National Hemophilia Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees. Wheeler:uniQure: Membership on an entity's Board of Directors or advisory committees; BioMarin: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk Inc: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees. Lentz:Novo Nordisk Inc.: Consultancy, Honoraria, Research Funding. Clausen:Novo Nordisk A/S: Employment. Cooper:Novo Nordisk Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal