Background:Despite the effectiveness of novel agents in treating CLL, single agent use requires prolonged administration which can lead to drug resistance, toxicity and considerable cost over time. Combinations may provide deeper prolonged remissions using defined treatment durations. Umbralisib (Umbra) is a novel, highly-specific PI3Kδ inhibitor and ublituximab (Ubli) is a chimeric monoclonal antibody targeting a unique epitope on CD20 and glycoengineered to enhance antibody dependent cellular toxicity. Combining these agents with the BCL2 inhibitor venetoclax (Ven) may prevent drug resistance (Choudhary, Cell Death Dis 2015), avoid tumor lysis syndrome (TLS) and achieve undetectable minimal residual disease (MRD). This phase 1/2 trial evaluates the safety and efficacy of Umbra + Ubli + Ven for 12 cycles followed by MRD evaluation in relapsed or refractory CLL patients (pts).
Methods:Pts received three 28-day cycles of Umbra daily along with Ubli, administered weekly during cycle 1, then once during cycles 2 and 3, followed by Umbra + Ven for 9 additional cycles. During the phase 1 study, dose levels including Umbra 600mg and 800mg were tested with Ubli 900mg and Ven, increased in standard fashion to 400 mg during cycle 4. The primary endpoint for phase 1 was safety; the primary endpoint for phase 2 was complete remission (CR) rate by iwCLL criteria. MRD negativity (<10-4by 8-color flow cytometry) was a key secondary endpoint with bone marrow and peripheral blood MRD negative pts stopping therapy after 12 cycles and other pts continuing on single agent Umbra.
Results: 21 pts have been treated to date: 9 in phase 1 and 12 in phase 2. Baseline demographics were as follows: male/female (12/9), median age 65 yrs (range 49-83), median prior therapies 2 (1-5). 9 pts had prior ibrutinib of which 4 were BTK inhibitor refractory. BTK resistance mutations were found in 2 pts. High risk genetic features included unmutated IGHV genes (11 pts), del17p or del11q (7 pts), TP53 mut (1 pt), NOTCH1 mut (4 pts) and SF3B1 mut (1 pt). Baseline TLS risk was high, medium and low risk in 2, 12 and 7 pts respectively.
During dose escalation, 3 pts were treated using Umbra 600mg and subsequently 6 pts using Umbra 800mg. No DLTs occurred, and the MTD was not reached. As such, the phase 2 dose used was Ubli 900 mg + Umbra 800 mg with venetoclax, undergoing ramp up to 400 mg.
For all pts treated to date, the most common AEs were (all causality and all grade; >20% of pts) infusion reactions (62%), thrombocytopenia (57%), neutropenia (52%), anemia (52%), fatigue (52%), ALT or AST increase (43%), nausea (33%), diarrhea (29%) and headache (24%). Grade 1 creatinine increase, hypocalcemia and hyperkalemia were observed in 12 (57%), 6 (29%) and 5 (24%) of pts respectively. These AEs predominately occurred outside of venetoclax ramp up. Grade 3/4 AE's occurring in ≥ 2 pts included neutropenia (n=4, 19%), infusion reaction (n=2, 10%) and thromboembolism (n=2, 10%). No grade ≥ 3 PI3Kδ-associated toxicities were noted (0 events of pneumonitis, colitis or grade 3/4 transaminitis). No events of TLS were observed. One pt discontinued study treatment due to grade 3 rash. All pts were converted to low TLS risk after 3 cycles of Umbra + Ubli, except for 1 pt who remained medium risk after discontinuing Ubli secondary to a grade 3 infusion reaction, allowing outpatient venetoclax initiation in all pts.
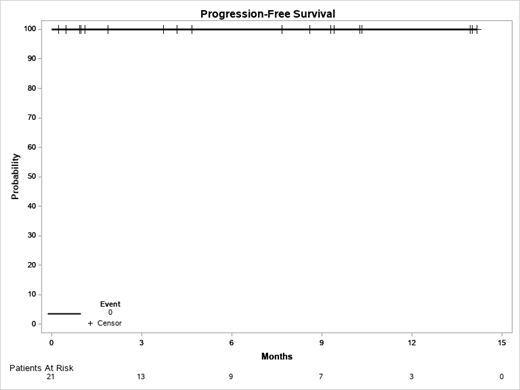
In evaluable pts, the overall response rate was 85% (11/13) after cycle 3, 100% (9/9) after cycle 7 and 100% (5/5) after cycle 12. Of the 5 pts who finished 12 cycles of therapy, a median reduction in nodal disease of 87% occurred resulting in 2 CRs and 3 PRs by iwCLL criteria. 4 pts have undetectable MRD (<0.01%) in both the peripheral blood and bone marrow and have stopped therapy. MRD was undetectable in peripheral blood and intermediate (0.01% - 1.0%) in the bone marrow for 1 pt who continues on Umbra. With a median f/u of 4.2 months (range 0.2-14.2), no pts have experienced disease progression. (Figure)
Conclusion: We established the phase 2 dose of Umbra + Ubli + Ven and demonstrated good tolerability in pts with relapsed or refractory CLL. Preliminary results suggest that this chemotherapy-free regimen can provide undetectable MRD after only 12 cycles, representing an effective treatment plan for this population. Ongoing enrollment is focused on pts who have relapsed after BTK inhibitors and a multi-center trial is planned to further develop the triplet regimen.
Barr:Gilead: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Verastem: Consultancy; Seattle Genetics: Consultancy; Celgene: Consultancy; Merck: Consultancy; Genentech: Consultancy; Astra Zeneca: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding. Hill:TG therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Research Funding; Kite: Consultancy, Honoraria; Amgen: Research Funding; Takeda: Research Funding; Seattle Genetics: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria; Celegene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Ma:Janssen: Consultancy, Speakers Bureau; Beigene: Research Funding; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Novartis: Research Funding; Xeme: Research Funding; Juno: Research Funding; Incyte: Research Funding; Kite: Consultancy; Genentech: Consultancy; Acerta: Research Funding; Bioverativ: Consultancy; Astra Zeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Research Funding; Gilead: Research Funding. Liesveld:Abbvie: Membership on an entity's Board of Directors or advisory committees; Onconova: Other: Data safety monitoring board. Sportelli:TG Therapeutics: Employment. Miskin:TG therapeutics Inc.: Employment, Equity Ownership. Weiss:TG Therapeutics: Employment. Friedberg:Acerta: Other: Data & Safety Monitoring Committee; Bayer: Honoraria, Other: Data & Safety Monitoring Committee. Zent:Astra Zeneca: Research Funding; Mentrik Biotech: Research Funding.
umbralisib and ublituximab as treatment of CLL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal