Introduction
Prognosis of elderly patients with newly diagnosed diffuse large B cell lymphoma (DLBCL) remains poor as compare to young patients. Co-morbidities and physiological organ function impairment often lead to non-manageable toxicities and limit optimal chemotherapy. A decreased dose of CHOP (doxorubicin 25mg/m2 on D1, cyclophosphamide 400mg/m2 on D1, vincristine 1 mg TD on D1, and prednisone 40mg/m2 D1 to D5) chemotherapy with a conventional dose of rituximab (R-miniCHOP) displays a good compromise between efficacy and safety in this population. Lenalidomide, an oral immunomodulatory drug, in combination with RCHOP (R2-CHOP) has an acceptable toxicity and a potential higher efficacy in the non-GCB subtype, more frequent in elderly patients. The SENIOR study evaluated the tolerance and efficacy of the R2-miniCHOP regimen in comparison to the standard R-miniCHOP in patients ≥80 years with newly diagnosed DLBCL.
Methods
SENIOR is a multicentric, phase III, open-label, randomized trial in patients aged of 80 years or more with non-previously treated CD20+ DLBCL, age-adjusted IPI= 0 to 3, and Ann Arbor stage II to IV. Eligible patients were randomized 1:1 to standard R-miniCHOP or R2-miniCHOP on a 21-days cycle for 6 cycles. Patients were stratified by CD10 expression and age (≤85 years old or > 85 years old). The first cycle of rituximab was delivered by IV at the dose of 375mg/m2 on D1, then subcutaneoulsy at the dose of 1400mg TD on D1 of cycles 2-6. In the R2-miniCHOP arm, lenalidomide was administered at a dose of 10 mg TD on D1 to D14 every 3 weeks. Patients in the experimental arm had to receive deep venous thrombosis prophylaxis. This trial included a pre-phase treatment of prednisone (60 mg/m2 per os, days -7 to -1) and vincristine (1 mg TD IV, at D -7)). The primary end point was overall survival (OS). The statistical plan was based on the hypothesis of an increase of 15% of the 2y-OS (59 %-> 74%) in favor of the experimental arm. Secondary endpoints were PFS (Progression-Free Survival), EFS (Event-Free Survival), duration of response, DFS (disease-free survival), response rate at the end of the treatment (registration number NCT02128061)
Results
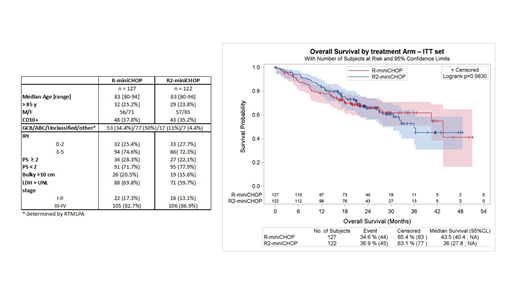
From August 2014 to September 2017, 249 patients were randomized (127 in R-miniCHOP arm and 122 in R2-miniCHOP arm). Median age was 83y (range 80-96). Baseline characteristics were balanced between the two arms (Table 1). 48 (19%) patients discontinued treatment (27 in R-miniCHOP and 21 in R2-miniCHOP), 27% for concurrent illness and 25% for progressive disease. In the safety set population (n = 241), the 6 planned cycles were delivered in 80% and 86% of patients in both arms. The % of planned dose for each R-miniCHOP compound was similar in both arms. 75% of patients received ≥ 75% of the planned dose of lenalidomide. Twenty (17%) patients experienced a dose reduction, 17 (85%) due to adverse events (AE). After a median follow up of 25.1 months, in an intention to treat analysis , R2-miniCHOP did not improve OS (2y-OS 66% in R-miniCHOP and 65.7% in R2-miniCHOP arm, p=.98, Figure 1), and no significant difference was observed based on stratified analysis on age or CD10 expression status. There was no significant difference in PFS, EFS, and DFS. ORR was 73% in R-miniCHOP arm and 83% in R2-miniCHOP arm. Deaths occurred in 42 and 43 patients in R-miniCHOP and R2-miniCHOP arm respectively, mainly due to lymphoma (60%). Four deaths, 2 in each treatment arm, were related to treatment toxicity. Grade 3-4 AE occurred in 53% of cases in R-miniCHOP arm and in 81% in R2-miniCHOP arm, with neutropenia in 17.7% vs 32.5%, infections 8.1% vs 13.7%, and pulmonary embolism (n=1, 0.8% vs n=7, 6%). Second primary malignancies occurred in 8 patients in R-miniCHOP arm and in 11 patients in R2-minCHOP arm. Both MNA and IADL scales correlated significantly with OS and PFS.
Conclusion
SENIOR study is the first prospective phase III trial in ≥80 years old patients with newly diagnosed DLBCL. Addition of lenalidomide to R-miniCHOP does not significantly improve OS irrespective to CD10 status and results in more adverse events. Rituximab delivered subcutaneously was safe and well tolerated. The overall 2y-OS of 66% was similar or higher to those reported in previously published LYSA trials (2y-OS = 59%, Peyrade et al. Lancet Oncol 2011; 2y-OS = 64.7%, Lancet Hematol 2017). Tumour Molecular characterisation are currently ongoing to identify patients that could benefit of R2-miniCHOP.
Oberic:Janssen: Honoraria; Roche: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Feugier:Roche,: Membership on an entity's Board of Directors or advisory committees, Other: Travel expense and grants; Gilead,: Membership on an entity's Board of Directors or advisory committees, Other: travel support and grants ; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: travel support and grants; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: travel support and grants; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: travel support, grants. Thieblemont:Celgene: Honoraria; Cellectis: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Research Funding; Gilead: Honoraria; Novartis: Honoraria; Kyte: Honoraria; Janssen: Honoraria. Salles:Roche, Janssen, Gilead, Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Amgen: Honoraria, Other: Educational events; BMS: Honoraria; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis, Servier, AbbVie, Karyopharm, Kite, MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Epizyme: Consultancy, Honoraria. Tilly:roche: Membership on an entity's Board of Directors or advisory committees; servier: Honoraria; merck: Honoraria; Gilead: Honoraria; Janssen: Honoraria; BMS: Honoraria; Karyopharm: Consultancy; Astra-Zeneca: Consultancy; Roche: Consultancy; Celgene: Consultancy, Research Funding. Haioun:servier: Honoraria; amgen: Honoraria; janssen cilag: Consultancy; takeda: Consultancy; novartis: Honoraria; celgene: Honoraria; gilead: Consultancy; celgene: Consultancy; roche: Consultancy. Jardin:amgen: Honoraria; Servier: Honoraria; celgene: Honoraria; roche: Honoraria; janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal