Introduction
Acalabrutinib was approved by the US Food and Drug Administration on October 31, 2017 for patients with MCL who have progressed on at least one prior therapy and is currently being evaluated in phase III trials for first-line MCL, first-line CLL, and relapsed/refractory (r/r) CLL. Additionally, acalabrutinib received guideline and compendia listing for use in MCL and CLL patients. There is a lack of information on the current treatment patterns with acalabrutinib in real world clinical practice. To that end, we conducted a descriptive analysis of the use of acalabrutinib in the real-world setting for MCL and CLL patients.
Methods
A retrospective cohort study was conducted using IQVIA's longitudinal prescription (LRx) database linked to the medical claims (Dx) database. Patients ≥18 years old with ≥1 claim for acalabrutinib in LRx between November 1, 2017 and April 30, 2019 were identified; the first claim was index date. Patients were also required to have ≥ 1 Dx claim between November 1, 2016 and April 30, 2019, and ≥ 12 months baseline (defined as ≥ 1 LRx claim and ≥ 1 Dx claim within 12 months pre-index and > 12 months pre-index). Patients were required to have ≥2 diagnoses of either MCL or CLL before index (without diagnosis of the other cancer) to create two mutually exclusive cohorts. Patients were excluded if they had data quality issues or evidence of clinical trial enrollment during the study period. Descriptive statistics were used to summarize baseline demographic and clinical characteristics overall and stratified by prior ibrutinib use. Results were described for MCL and CLL cohorts separately.
Results
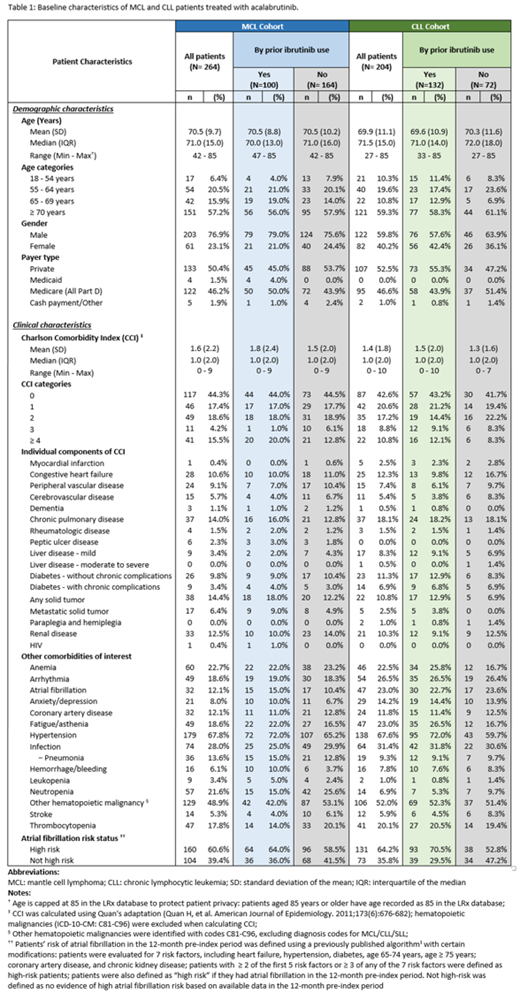
A total of 264 MCL and 204 CLL patients treated with acalabrutinib were identified in the study. For both cohorts, the majority (57.2% MCL / 59.3% CLL) were 70 years or older, with a median (interquartile range [IQR]) age of 71.0 (15.0), mean (standard deviation [SD]) of 70.5 (9.7) for MCL and median age of 71.5 (15.0), mean 69.9 (11.1) for CLL patients (Table 1). 76.9% of MCL and 59.8% of CLL patients were male, and over half were commercially insured (50.4% MCL / 52.5% CLL). In the 12-month pre-index period, MCL and CLL patients had a mean (SD) Charlson Comorbidity Index (CCI, excluding hematologic malignancies) of 1.6 (2.2) and 1.4 (1.8), respectively; hypertension (67.8% MCL / 67.7% CLL), other hematologic malignancies (48.9% MCL / 52.0% CLL), and infection (28.0% MCL / 31.4% CLL) were the most common comorbidities. Other common (i.e., ≥ 20%) conditions included anemia and neutropenia for the MCL cohort, and arrhythmia, fatigue/asthenia, atrial fibrillation (A-fib), anemia and thrombocytopenia for the CLL cohort. Over 60% of MCL and CLL acalabrutinib-treated patients were defined as high risk of A-Fib (Chyou, Hunter et al. 2015). In terms of prior Bruton's Tyrosine Kinase-inhibitor (BTKi) treatment, 37.9% of MCL and 64.7% of CLL patients had prior ibrutinib use. More prior ibrutinib users were defined as high risk A-Fib status at time of acalabrutinib start than patients without prior ibrutinib use, and prior ibrutinib users had a higher frequency of several comorbidities (e.g., hypertension, fatigue/asthenia; Table 1).
Conclusions
With the relatively recent approval, this is the first analysis of acalabrutinib use in clinical practice and description of characteristics of MCL and CLL patients being treated with this medication in the real world. Before initiating acalabrutinib, approximately one-third of MCL patients and two-thirds of CLL patients received ibrutinib (reasons for discontinuing ibrutinib were not explored). Although the overall comorbidity score was similar between patients with and without prior BTKi exposure, BTKi-naïve patients had a slightly lower frequency of high-risk A-Fib status at acalabrutinib initiation. Future studies are warranted to evaluate the treatment patterns of acalabrutinib and outcomes in the real-world setting.
Ryan:AstraZeneca: Employment, Equity Ownership. Burudpakdee:IQVIA: Consultancy. Zhao:IQVIA: Consultancy. Le:AstraZeneca: Employment, Other: Stocks. Near:IQVIA: Consultancy.
Acalabrutinib is an oral inhibitor of Bruton's Tyrosine Kinase. It was approved by the US Food and Drug Administration on October 31, 2017 for patients with mantle cell lymphoma (MCL) who have progressed on at least one prior therapy and is currently being evaluated in phase III trials for first-line MCL, first-line chronic lymphocytic leukemia (CLL), and relapsed/refractory CLL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal