INTRODUCTION
Peripheral T cell lymphomas (PTCL) are a rare and heterogenous group of aggressive non-Hodgkin lymphomas (NHL) that develop from mature T- and natural killer cells. They comprise approximately 10% of all newly diagnosed cases of NHL in Western populations. The current study characterizes real-world treatment patterns and outcomes among patients with PTCL in France and the United Kingdom (UK) in the first-line (1L) setting.
METHODS
A retrospective medical chart review study was undertaken at geographically disparate clinical sites in France and UK, where treating physicians were responsible for patient selection and data collection via structured case report forms. Adults (≥18 years) with newly diagnosed with PTCL between January 1, 2014 and December 31, 2016 were randomly selected for inclusion in the study. Eligible patients had received at least 1L treatment (1LT) for PTCL and had available clinical data for ≥1 year after PTCL diagnosis or until death. Patients enrolled in a clinical trial for 1L PTCL at any time between diagnosis and end of follow-up or who had any prior unresolved malignancy within 5 years of PTCL diagnosis were excluded. Demographic, clinical, and immunophenotypic/cytogenetic profiles of PTCL patients were assessed at diagnosis. Treatments received and best response as reported by the treating clinician (categorized as complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD]) at the end of 1LT are described. Data from both countries were pooled for the current analysis.
RESULTS
Overall, 32 haematologist/oncologists (France, 15; UK, 17) with a median of 12.5 years of clinical practice participated in the study. Most physicians in France practiced at a university hospital or a regional hospital centre (73.3%). In the UK, most physicians practiced at a specialist cancer/ tertiary referral treatment centre (47.1%). A total of 109 patients (France, 53; UK, 56) received at least 1L PTCL treatment during the study period. The median age at diagnosis was 63 years (range, 19 to 84 years), with a male predominance (57.8%). Eighty percent of patients had an ECOG performance status of 0-1 at diagnosis. B symptoms were present in 59.6% and extranodal disease was present in 35.8% patients at diagnosis. Most patients (72.5%) had stage III or IV disease and (64 of 102) 62.75% were classified as intermediate risk (score of 2-3) as per the International Prognostic Index. The most common histological subtypes were PTCL-not otherwise specified (NOS) (37.6%), angioimmunoblastic T-cell lymphoma (29.4%), and systemic anaplastic large cell lymphoma (20.2%). The median duration of follow-up was 34 months from the start of 1L PTCL treatment. Of those tested, 60.4% patients were positive for CD30 (information on threshold was not reported). Only 19.3% patients underwent testing for any cytogenetic marker. During follow-up, 28.4% of patients had died.
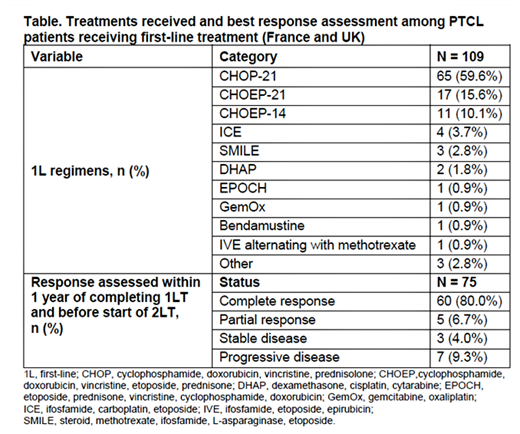
In the 1L setting, 59.6% and 26.6% patients were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) and with CHOEP/CHOEP‐like regimens (Table). Median duration of 1LT was 4.9 months. Stem cell transplant was undertaken in 29.4% patients as part of 1L treatment, of which 84.4% were autologous. PET-CT was used for response assessment in 56% of patients. Best response within 1 year of completing 1LT and before start of 2L treatment was assessed in 75/109 [68.8%] patients (Table); of whom 80% (60 patients) were reported to have a complete response (Table), and about 39% relapsed (within the first year) and went on to 2L treatment. The remaining 27 patients had best response assessed prior to completion of 1LT. Brentuximab vedotin (19%) and GemOx (19%) were the most common 2L treatments.
CONCLUSIONS
This retrospective observational study describes treatment patterns and key clinical outcomes among patients receiving 1L PTCL treatment in France and the UK. CHOP-based regimens were used to treat 59.6% of the study patients. Understanding distribution of PTCL subtypes and outcomes of treatment in routine clinical practice is complementary to data derived from clinical trials and crucial to facilitate improvement of survival outcomes through the introduction of novel therapies for this challenging group of rare malignancies.
Fox:Janssen: Consultancy; Adienne: Other: Travel Support; Takeda Pharmaceuticals: Consultancy; Atara Biotherapeutics: Consultancy; AbbVie: Consultancy; Gilead: Consultancy; Celgene: Consultancy; Sunesis: Consultancy. Ashaye:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd: Other: I am a paid employee of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd. Shah:Pfizer: Research Funding; Merck: Research Funding; Bayer: Research Funding. Dalal:Millennium Pharmaceuticals (Takeda): Other: I am a paid employee of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd. Truemper:Nordic Nanovector: Consultancy; Mundipharma: Research Funding; Janssen Oncology: Consultancy; Roche: Research Funding; Seattle Genetics, Inc.: Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal