Introduction
Neutropenic fever following high-dose chemotherapy and autologous stem cell transplantation (ASCT) is a common (incidence 63-100%) and potentially life-threatening complication. Recommended time to antibiotic (TTA) administration is within 1 hr of fever onset with delays associated with significant morbidity, prolonged hospitalization, and mortality. Standard of care guidelines emphasize patient self-monitoring for fever, with instructions to seek immediate medical attention if body temperature (temp) reaches 100.4°F or higher. In this study, we evaluated if a novel wearable, continuous temp monitor, tPatch, could reliably estimate core body temp and detect fever in an outpatient setting following ASCT. Additionally, we gathered preliminary data to explore early detection and prediction of clinically relevant temp rise in this clinical setting.
Methods
Patients (N = 86) with hematologic malignancies (62% multiple myeloma) who underwent high-dose chemotherapy followed by ASCT at Mayo Clinic, MN were prospectively enrolled between June 2018 and March 2019. Patients (82% male) wore an axilla-placed tPatch continuously for 7 days in an outpatient setting during the post ASCT period and were asked to record self-measured oral temp in 3-4 hr intervals daily using a standardized thermometer after appropriate training . Patients followed standard of care procedures with daily clinic assessment of temp, blood counts, and vital signs. An optional patient questionnaire was given at end-of-study. A model was trained using both patient- and clinic-assessed oral temp measures to estimate core temp from 2 sensors on the tPatch device. Core temp estimates and trends were then compared to patient- and clinic-assessed measurements. Fever was defined as a temperature ≥100.4°F for at least 1 hr.
Results
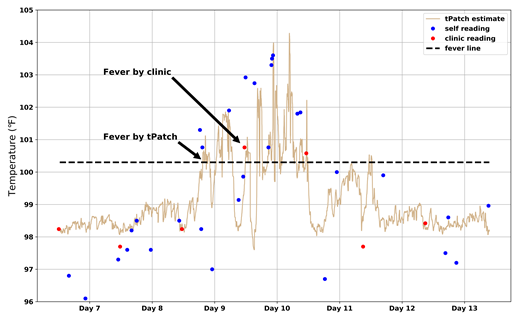
When compared to all oral temp reads, the tPatch estimated core temp within 0.03 ± 0.7℉. Among the 86 patients, clinic-assessed fever incidence was 29.4% while tPatch-assessed incidence was 58.8%. Using all clinic-recorded temp readings as "ground truth," the sensitivity and specificity of the tPatch algorithm in detecting fevers were 88% and 86%, respectively, while patient self fever detection sensitivity was 62% and specificity 93%. With "fever episode" defined as a temp ≥100.4°F for at least 1 hr, tPatch detected 9.6 times the number of fever episodes vs. clinic reads. The average lead time of tPatch detection of clinic-recorded fevers was 3.7 hours. In 25% of all intervals between clinic temp readings, either tPatch or patients detected at least 1 fever episode.
The tPatch was well-tolerated, the only adverse events reported were grade 1 skin irritation and discomfort in 4 (5%) patients. Of 65 patients who completed the survey, 95% reported the tPatch as "quite" or "somewhat" comfortable and 94% stated no difficulty in using the tPatch. Exploration of tPatch temp trends over various time intervals for use in fever prediction is ongoing.
Conclusions
Patient self-monitoring of temp has low sensitivity and is not feasible for long intervals of time (e.g., overnight). Continuous temperature monitoring by a wearable device overcomes these challenges and has the potential to improve early detection and consequently shorten time to antibiotic initiation. A follow-up randomized study is planned to assess the clinical benefits of continuous temp monitoring through patient and clinician alerts triggering early clinical intervention for febrile neutropenia.
Vera-Aguilera:Verily Life Sciences: Research Funding. Haji-Abolhassani:Verily Life Sciences: Employment. Kulig:Verily Life Sciences: Employment. Heitz:Verily Life Sciences: Employment. Paludo:Verily Life Sciences: Research Funding; Verily Life Sciences: Research Funding; Celgene: Research Funding; Celgene: Research Funding. Ghoreyshi:Verily Life Sciences: Employment. Scheevel:Verily Life Sciences: Research Funding. Schimke:Verily Life Sciences: Research Funding. Markovic:Verily Life Sciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal