Introduction: Novel triplet therapies (tx) for relapsed/refractory multiple myeloma (RRMM) have improved outcomes and extended survival. However, the use of multi-agent tx over an extended period increases the tx-related symptom burden and impacts health-related quality of life (HRQoL). Therefore, highly effective tx that preserve HRQoL are needed. The randomized phase 2 ELOQUENT-3 study (NCT02654132) demonstrated that addition of elotuzumab (elo) to pomalidomide and dexamethasone (EPd) resulted in a 46% reduction in the risk of progression/death vs Pd, without affecting HRQoL, in patients (pts) with RRMM for whom lenalidomide (len) and a proteasome inhibitor (PI) had failed (Dimopoulos et al. N Engl J Med 2018; Weisel et al. ASH 2018). Here, we present pt-reported outcomes (PROs) with EPd vs Pd after extended follow-up (FU) of ELOQUENT-3.
Methods: PROs were an exploratory endpoint, assessed using the 3-level EuroQoL 5 Dimensions Questionnaire (EQ-5D-3L) and the MD Anderson Symptom Inventory MM module (MDASI-MM). The EQ-5D-3L includes a global health visual analog scale (VAS) and utility index (UI); the MDASI-MM measures total symptom severity (13 core items plus 7 MM items) and symptom interference (6 items). EQ-5D-3L UI and VAS scores range from −0.59 to 1 and 0 to 100, respectively, with minimally important differences (MIDs) of 0.08 and 7. MDASI-MM scores range from 0 to 10; MIDs were based on the standard error of the mean for subscales. Higher scores indicate better health for EQ-5D-3L, but more severe symptoms for MDASI-MM. PRO data were collected at baseline (BL), at the start of every 28-d tx cycle, at the end of tx, and during FU. All randomized pts with BL and ≥1 post-BL assessment were included in the PRO analysis. Completion rates and changes from BL scores were evaluated descriptively; completion rates from the 'expected population' did not include pts who had died or discontinued. Longitudinal analyses of change from BL used mixed effects models. First deterioration/improvement was defined as the first change from BL that was ≥responder definition threshold.
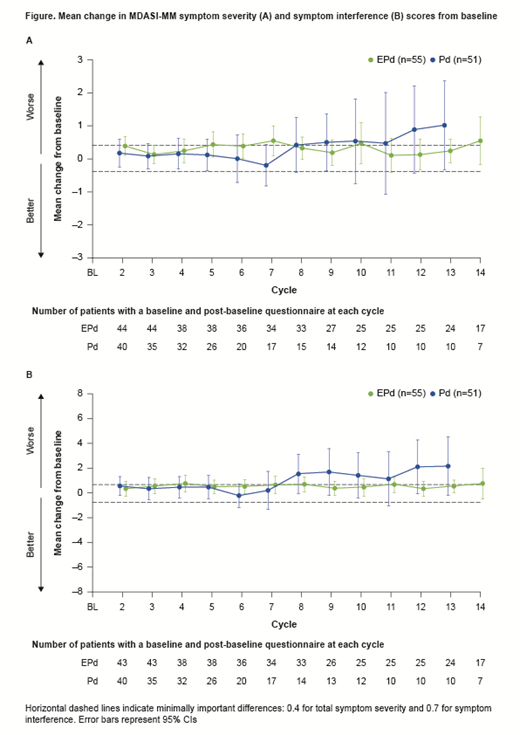
Results: Of 117 randomized pts, 106 (EPd n=55; Pd n=51) had BL and ≥1 post-BL assessment and were included in PRO analyses (database lock, Nov 2018; minimum FU, 18.3 mo). BL characteristics of the PRO population were generally balanced between arms and representative of the entire study population. PRO completion rates from the expected population were ≥79% and ≥96% for the MDASI-MM and EQ-5D-3L, respectively, for all on-tx timepoints. Although completion rates between arms were similar throughout, between-tx HRQoL analysis was not feasible after Cycle 13 due to low Pd pt numbers. Mean BL scores were similar between arms: EPd vs Pd MDASI-MM total symptom severity, 1.5 vs 1.6; symptom interference, 2.5 vs 2.3; EQ-5D-3L UI, 0.70 vs 0.68; VAS, 65.6 vs 69.2.
In the EQ-5D-3L UI, neither tx arm had a clinically meaningful deterioration (CMD); in the VAS, there was a CMD in the Pd arm only. In MDASI-MM total symptom severity, there was a CMD in both arms (Figure). In MDASI-MM symptom interference, there was a CMD in both arms at some timepoints (Figure). However, Pd sample sizes were small for the MDASI-MM (n≤15).
Longitudinal analyses demonstrated no clinically meaningful differences between arms for EQ-5D-3L UI and VAS or MDASI-MM total symptom severity and symptom interference or the items of pain, fatigue, or bone aches. There were no statistically significant differences in time to deterioration between arms for EQ-5D-3L UI or VAS. However, there was a trend towards a reduction in the risk of deterioration in the EQ-5D-3L VAS for pts receiving EPd vs Pd (HR 0.70; 95% CI 0.43-1.14; p=0.110). Median time to deterioration was generally similar between arms across the MDASI-MM subscales. Hospitalizations were similar between EPd (32 pts [53%]) and Pd arms (31 pts [54%]). Mean duration of hospitalization was 9.9 d with EPd and 12.9 d with Pd.
Conclusions: HRQoL was similar between pts who received EPd and Pd in ELOQUENT-3, demonstrating the addition of elo to Pd did not impair HRQoL. These pt-reported findings complement extended FU data that demonstrated EPd gave clinically meaningful improvements in survival without increasing toxicity, further supporting the use of EPd in pts with RRMM after failure of len and a PI. Further PRO analysis in a larger study is warranted.
Study support: BMS. Writing support: Adam Gill, Caudex, funded by BMS.
Weisel:Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Juno: Consultancy. Paner:Rush University Medical Center: Employment; Dova: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Cellectar: Consultancy, Honoraria; Cellectar: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria. Taylor:Adelphi Values: Employment, Other: I am an employee of Adelphi Values, a consulting firm who has received payment from Bristol-Myers Squibb for statistical data analysis in Bristol-Myers Squibb's trials. Cocks:Amgen: Consultancy; BMS: Consultancy; Adelphi Values: Employment; Celgene Corporation: Consultancy; Endomag Ltd.: Consultancy. Popa-McKiver:Bristol-Myers Squibb: Employment. Chen:Bristol-Myers Squibb: Employment. Cavo:Janssen, Celgene: Other: Travel Accommodations; Celgene, Janssen, Amgen, BMS, Abbvie, Takeda: Honoraria; Janssen, Celgene: Speakers Bureau; Janssen, Celgene, Amgen, Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal