Background: Ibritumomab tiuxetan (IbT) is a radioimmunoconjugate linking rituximab with 90Y isotope. IbT is approved for treatment of relapsed/refractory indolent B-cell lymphomas, or for consolidation after chemotherapy in follicular lymphoma (FL), yet its use has been limited by concern about treatment-related myeloid neoplasms (tMDS/AML: acute myeloid leukemia and myelodysplastic syndrome). The risk of tMDS/AML may differ among older patients who experience significant competing mortality. Our objective was to use population-based data to examine real-life patterns of use of IbT, and associated incidence of tMDS/AML among older patients with B-cell lymphomas.
Methods: From the SEER-Medicare registry, which covers about 28% of the United States population, we selected fee-for-service Medicare beneficiaries >60 years [y] old, with B-cell lymphomas diagnosed between 2001-2015, who received systemic therapy. We identified the use of IbT, purine analogues, alkylating agents, and rituximab. We defined tMDS/AML as myeloid neoplasms diagnosed >2 months from the start of first-line therapy for lymphoma, or use of tMDS/AML-directed chemotherapy. We estimated incidence rate and incidence rate ratio (IRR) for tMDS/AML after IbT exposure using a time-split survival model. By matching IbT recipients with non-recipients according to age, sex, lymphoma histology, and observation time from the start of lymphoma therapy, we compared the cumulative incidence function of tMDS/AML using competing risk models stratified by matched groups. We fitted the univariate model, and model adjusting for prior exposure to purine analogues or alkylators.
Results: Among 43,945 Medicare beneficiaries (median age of 76 y) treated for diffuse large B-cell (DLBCL, 48%), FL (22%), mantle cell (7%), marginal zone (10%), or unspecified B-cell lymphoma (14%), 428 (1%) received IbT, at median 18 months from first-line chemotherapy (interquartile range [IQR], 8-34). IbT was used most frequently in FL (50%) and DLBCL (23%), and was applied as first-line treatment in 30 patients (7%).
With median follow-up of 8.5 y, 618 cases of tMDS/AML were recorded (15 among IbT recipients, and 603 among non-recipients). Median latency from the start of first-line lymphoma therapy to tMDS/AML was 38 months (IQR, 18-65). The cumulative incidence rate of tMDS/AML after IbT exposure was 2.8% at 5y (95%CI, 1.2-4.4) and 3.9% at 10y (95%CI, 1.8-6.0). No tMDS/AML was observed among patients who received IbT as first-line treatment, before chemotherapy exposure.
In the time-split survival analysis, the incidence of tMDS/AML was 0.39 per 100 person-years (95%CI, 0.18-0.60) without IbT exposure, and 1.02 (95%CI, 0.56-1.71) after IbT exposure, with an IRR of 3.22 (95%CI, 1.80-5.26). There was no significant heterogeneity in the IRR by lymphoma histology (Mantel-Haenszel test of homogeneity, P=.47).
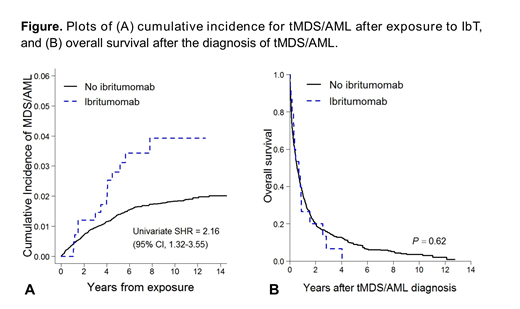
In the matched analysis (Fig A), the cumulative incidence of tMDS/AML was significantly higher among patients receiving IbT (subhazard ratio [SHR], 2.16; 95%CI, 1.32-3.55). However, the association became non-significant after adjusting for prior exposure to myelotoxic chemotherapy (SHR, 1.58; 95%CI, 0.93-2.68). Prior exposures to a purine analogue (SHR 3.83; 95%CI, 2.96-4.95) or an alkylator (SHR, 1.39; 95%CI, 1.11-1.75) remained significantly associated with increased risk of tMDS/AML. Median overall survival after tMDS/AML diagnosis was 0.6y (95%CI, 0.5-0.7), and it did not differ according to IbT exposure status (P=.62; Fig B).
Conclusions: The cumulative incidence of secondary tMDS/AML in this large population-based cohort is similar to reports from clinical trials (Czuczman et al. J Clin Oncol. 2007), and lower than in some prior observational series (Epperla N, et al., Br J Haematol 2017). Our results support the use of IbT for older patients with indolent lymphomas. Exposure to IbT was associated with an increased incidence of tMDS/AML, but it was no longer significant after adjustment for prior exposure to myelotoxic chemotherapy. This result suggests that the increased incidence of tMDS/AML is a cumulative effect of IbT with other myelotoxic agents. Caution should thus be exercised when using IbT after exposure to chemotherapy. The fact that no tMDS/AML were observed in patients receiving IbT as first line therapy suggests that earlier use of IbT may be preferable from the point of view of the tMDS/AML risk.
Olszewski:Genentech: Research Funding; TG Therapeutics: Research Funding; Spectrum Pharmaceuticals: Research Funding; Adaptive Biotechnologies: Research Funding.
Ibritumumab tiuxetan - for treatment of any B-cell lymphoma in any setting
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal