Introduction: The phase 3 international, randomized, double-blind ROBUST clinical trial (NCT02285062) compared progression-free survival between patients with previously untreated ABC-type DLBCL treated with placebo plus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (placebo-R-CHOP) versus lenalidomide + R-CHOP (R2-CHOP) for 6 21-day cycles. HRQoL, as measured by the Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) and the EuroQoL 5-dimension 3-level (EQ-5D-3L) patient-reported questionnaires, was evaluated as a secondary endpoint for the 2 treatment arms.
Methods: For each treatment group, FACT-Lym and EQ-5D-3L scores were measured at baseline (BL), mid-, end-of-treatment (EOT), and post-treatment, in 12-week intervals from Week 34 onward. Patients were evaluated if they had completed BL and ≥ 1 post-BL calculable FACT-Lym subscale measurements. Non-inferiority (NI) of R2-CHOP versus placebo-R-CHOP was measured for 4 key FACT-Lym subscales: Physical Well-Being (PWB), Additional Concerns (AC), Functional Well-Being (FWB), and Trial Outcome Index (TOI). NI was assessed at EOT (typically 3-4 weeks after completing Cycle 6) and at post-treatment follow-up at Week 34 by analysis of covariance (ANCOVA). The NI margins were prespecified as 1 less than the higher published minimal important difference (MID) for each subscale. Since a range of MIDs have been published, a more conservative value was also used as a sensitivity analysis. Time to improvement (TTI), using published responder definitions, was also assessed for the FACT-Lym subscales. EQ-5D-3L scores were summarized descriptively.
Results: Demographic and baseline disease characteristics were balanced across the 2 treatment arms (N = 285 per arm). The percentage of patients who completed the FACT-Lym out of the expected population remained > 85% at all time points with > 50 patients still on the study in each treatment arm up to Week 142. Completion rates and reasons for missing assessments were balanced across the 2 treatment arms.
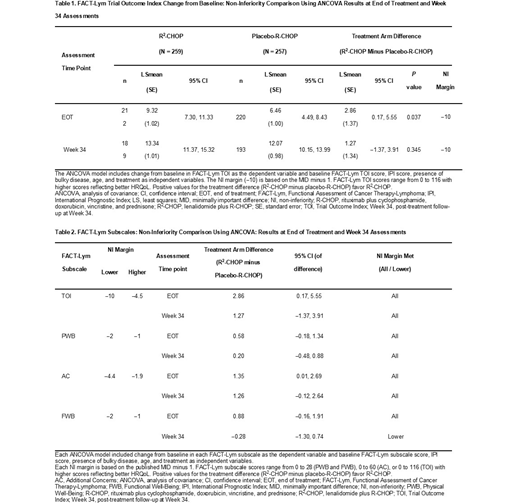
FACT-Lym subscale scores were generally higher after EOT, similar for both treatment groups, and mean changes from BL were close to or above MID (where defined), except for the Social/Family Well-Being subscale. Mean differences in FACT-Lym TOI between treatment arms are shown in Table 1. As positive treatment differences favor R2-CHOP and -10 was the predefined NI margin, NI was demonstrated for the FACT-Lym TOI subscale at both EOT and Week 34 assessments. Superiority of R2-CHOP was demonstrated at EOT as the confidence interval (CI) lies completely above 0 (P < 0.04); however, this difference was not clinically meaningful. Furthermore, NI was also demonstrated when using the sensitivity analysis margin of -4.5.
ANCOVA models were also fitted for the other key subscales of PWB, AC and FWB (Table 2). NI was demonstrated for all NI margins at both time points for the PWB and AC subscales. The FACT-Lym FWB subscale demonstrated NI for both margins at EOT and Week 34 for the primary NI margin of -2 based on the MID minus 1, but did not demonstrate NI when an NI margin of -1, based on an alternative published MID, was used.
The TTI curves and associated summary statistics were similar for the 2 treatment arms, with a median TTI for the FACT-Lym TOI of 21 weeks (95% CI 15-22) for patients treated with R2-CHOP and 18 weeks (95% CI 15-22) for patients treated with placebo-R-CHOP.
Mean EQ-5D-3L visual analogue scale (VAS) and Utility Index (UK weights) change from baseline scores were higher in both treatment arms and the mean changes were above, or close to, the published MID (7 for VAS, 0.1 for Utility Index) at EOT assessments.
Prespecified sensitivity and subgroup analyses supported the conclusions from the primary HRQoL analyses.
Conclusions: The predefined NI levels were met for the 4 key FACT-Lym subscales. Similar results were observed across the other HRQoL assessments, sensitivity, and subgroup analyses. Adding lenalidomide to the R-CHOP regimen did not adversely affect the HRQoL of patients with DLBCL as measured by FACT-Lym and EQ-5D-3L. These data serve as a benchmark for future clinical trials combining next-generation immunomodulatory drugs with standard R-CHOP to improve clinical efficacy while maintaining HRQoL in patients with DLBCL. HRQoL analyses should become an integral part of future clinical trials of novel combination regimens in DLBCL.
Chiappella:Roche: Speakers Bureau; Teva: Speakers Bureau; Servier: Other: advisory board, Speakers Bureau; Janssen: Other: advisory board, Speakers Bureau; Celgene: Other: advisory board, Speakers Bureau. Cocks:Adelphi Values: Employment; Amgen: Consultancy; BMS: Consultancy; Celgene Corporation: Consultancy; Endomag Ltd.: Consultancy. Greenwood:Adelphi Values: Employment. Williams:Adelphi Values: Employment. Scott:Celgene: Consultancy; Roche/Genentech: Research Funding; NanoString: Patents & Royalties: Named inventor on a patent licensed to NanoSting [Institution], Research Funding; Janssen: Consultancy, Research Funding. Yamamoto:SymBio: Research Funding; Ono: Consultancy, Honoraria, Research Funding; Otsuka: Honoraria; Sanofi: Honoraria; Astra-Zeneca: Consultancy, Research Funding; Sumitomo Dainippon: Honoraria; Chugai: Consultancy, Honoraria, Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Gilead Sciences: Research Funding; Solasia Pharma: Research Funding; Bayer: Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding; ARIAD: Research Funding; Pfizer: Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Kyowa Kirin: Honoraria; MSD: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Meiji Seika Pharma: Consultancy, Honoraria; HUYA/IQVIA Services Japan: Consultancy, Honoraria; Novartis: Honoraria, Research Funding; Incyte: Research Funding; Takeda: Consultancy, Honoraria, Research Funding. Jurczak:Bayer: Research Funding; Takeda: Research Funding; Gilead: Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Incyte: Research Funding; Celgene Corporation: Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celtrion: Research Funding; Novo Nordisk: Research Funding; Servier: Research Funding; TG Therapeutics: Research Funding. Özcan:Sanofi: Other: Travel support; Abdi Ibrahim: Other: Travel support; BMS: Other: Travel support; Takeda: Honoraria, Other: Travel support, Research Funding; Archigen: Research Funding; Roche: Other: Travel support, Research Funding; Bayer: Research Funding; Janssen: Other: Travel support, Research Funding; Celgene Corporation: Research Funding, Travel support; AbbVie: Other: Travel support, Research Funding; MSD: Research Funding; Novartis: Research Funding; Jazz: Other: Travel support; Amgen: Honoraria, Other: Travel support. Belada:Roche: Consultancy; Gilead Sciences: Consultancy; Takeda: Consultancy. Margunato-Debay:Celgene Corporation: Employment, Equity Ownership. Czuczman:Celgene Corporation: Employment, Equity Ownership. Zhai:Celgene Corporation: Employment, Equity Ownership. Braverman:Celgene Corporation: Employment. Vitolo:Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; F. Hoffmann-La Roche: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Nowakowski:Selvita: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding; MorphoSys: Consultancy, Research Funding; Curis: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech, Inc.: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal