Introduction: Pyruvate kinase (PK) deficiency is a rare, congenital autosomal recessive hemolytic anemia managed with supportive treatments, including transfusion, splenectomy, and iron chelation. Disease-directed treatments, including a small molecule PK activator and gene therapy, are currently in development. No disease-specific patient-reported outcome (PRO) measures have been validated for use in this patient population. The objective of this initiative was to develop PRO measures for assessing symptoms and impacts of PK deficiency and compare them to existing, non-disease-specific measures previously recommended for use in this disease area.
Methods: A targeted literature review was conducted to inform the development of a preliminary hypothesized conceptual framework to identify signs, symptoms, and impacts commonly experienced by patients with PK deficiency and to inform the direction and content of interviews with such patients. Concept elicitation interviews were conducted with 21 adults with PK deficiency from the US, Netherlands, and Germany. Draft items were then tested in cognitive interviews with 20 adults with PK deficiency to further establish content validity and revised based on the results. A comparison was conducted between concepts included in the newly developed PK deficiency disease-specific measures and the domain structure and item concepts included in the EORTC QLQ-C30 and SF-36v2 to evaluate the extent of differences and conceptual overlap with instruments that had previously been recommended in this population. Specific attributes compared included face validity (i.e., conceptual coverage and inclusion of proximal symptoms and/or impacts) and measurement characteristics (i.e., item wording, recall, and response options).
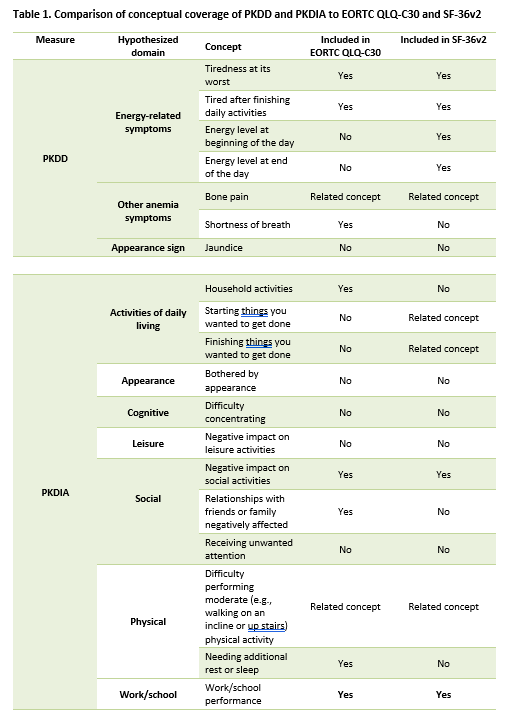
Results: Two measures, the PK Deficiency Diary (PKDD), a 7-item measure of the core signs and symptoms of PK deficiency, and PK Deficiency Impact Assessment (PKDIA), a 14-item measure of the impacts of PK deficiency on patients' HRQoL, were developed. A comparison of the newly drafted measures to the EORTC QLQ-C30 and SF-36v2 demonstrated minimal similarities in concepts, domains, item wording, and recall period. Of the 7 concepts in the PKDD, only 3 were common to the EORTC QLQ-C30, 4 were common to the SF36v2, and 2 were related but did not match exactly (i.e., "bone pain" and "pain"). Of the 12 distinct concepts in the PKDIA, only 5 were common to the EORTC QLQ-C30, 2 were common to the SF-36v2, and 3 were related but did not match exactly (i.e., difficulty starting things, difficulty finishing things, and difficulty performing moderate physical activity).
Conclusions: This research demonstrates that the EORTC-QLQ-C30 and SF-36v2 lack the appropriate conceptual relevance and coverage of disease-specific signs, symptoms, and impacts most relevant and burdensome to patients with PK deficiency. The newly developed PKDD and PKDIA may be useful tools in clinical trials in patients with PK deficiency. Psychometric validation of these measures is currently underway.
Salek:Pfizer: Honoraria, Speakers Bureau; Merck: Consultancy; Agios Pharmaceuticals, Inc.: Consultancy, Honoraria. Boscoe:Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Evans:Agios Pharmaceuticals, Inc.: Consultancy, Research Funding. Egan:Agios Pharmaceuticals, Inc.: Consultancy, Research Funding. Wells:Agios Pharmaceuticals, Inc.: Consultancy, Research Funding. Piantedosi:Agios Pharmaceuticals, Inc.: Employment. Grace:Agios Pharmaceuticals, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding. Storm:Agios: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal