BACKGROUND and OBJECTIVE: Tumor lysis syndrome (TLS), a potentially fatal oncologic complication, can have a significant clinical and economic impact to patients and the healthcare system. The aim of this analysis was to examine healthcare resource utilization in patients treated with rasburicase for the management of tumor lysis in the outpatient versus inpatient setting.
METHODS: Adult patients were selected from the Integra Connect Database (IC) if they were treated with rasburicase between January 1, 2017 and March 31, 2019. The IC database comprises clinical and financial records for more than 750,000 community oncology patients, including 25,000 active Oncology Care Model participants representing approximately 20% of the unique beneficiaries in this Medicare model. Patients treated in the outpatient setting were divided into 3 groups: Primary Prophylaxis- treatment with rasburicase administered days 0-2 of chemotherapy (Group A), Early Reactive- rasburicase administered days 3-5 of chemotherapy (Group B), and Late Reactive- rasburicase administered after day 5 of chemotherapy (Group C). Inpatients were divided into 2 groups: Inpatient TLS Treatment- patients admitted and treated for TLS with rasburicase and who had not received rasburicase as part of their outpatient chemotherapy regimen (Group D) and Inpatient Chemotherapy- patients admitted for chemotherapy who were given rasburicase (Group E). Demographic, clinical characteristics including tumor types, lab values, and dose information were collected. Total cost of rasburicase was calculated as mean drug cost per patient. All variables were summarized descriptively as mean (SD), median (min and max) or counts (percentages).
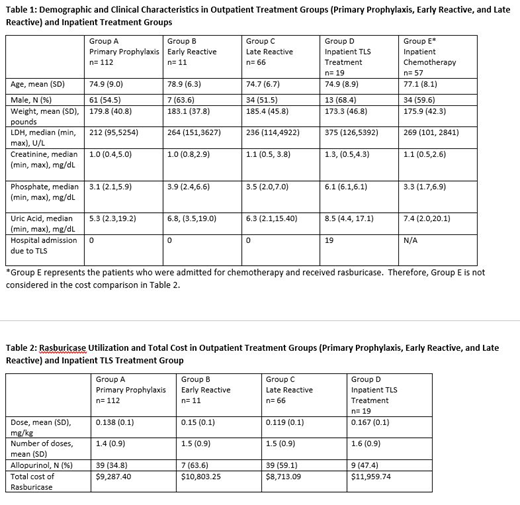
RESULTS: A total of 265 patients treated with rasburicase were included in the analysis. Of those, 189 patients received rasburicase in the outpatient setting vs 76 in the inpatient setting. None of the 189 patients in Groups A, B, and C who received outpatient rasburicase required admission due to TLS. Patient demographic and clinical characteristics, as well as rasburicase utilization, were similar between cohorts (Table 1 and Table 2). Our results show that while 54% of patients in Groups B, C, and D were initially treated with allopurinol, these patients were switched to rasburicase, indicating failure of allopurinol alone. The total cost of rasburicase trended lower in the outpatient vs inpatient setting ($9,287 vs $11,959). However, a more appropriate comparator to this cost is the published data for charges incurred for inpatient treatment of TLS, shown to be $151,9171
CONCLUSIONS: TLS continues to impact patients with diagnoses and chemotherapy regimens at intermediate and high risk for development of this syndrome. This study demonstrates that allopurinol is frequently inadequate and replacement with rasburicase is needed. We found rasburicase to be effective from both a clinical and a cost perspective in preventing TLS-related hospitalization. While rasburicase is most efficiently employed as primary prevention, close monitoring of patients allows effective reactive utilization evidenced by none of the outpatients in this study who received rasburicase were admitted for TLS. This study highlights the opportunity for greater utilization of rasburicase in the outpatient setting, as a means to lower the total cost of care.
1. Pathak et al. Blood. 2017; 130:3390
McBride:teva: Consultancy; Sandoz: Consultancy; Sanofi Genzyme: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal