Introduction: As the first in a new class of substitutive, non-factor hemophilia A therapy, acceptance of emicizumab by patients with hemophilia is unclear. Impact of emicizumab on treatment adherence, pursuit of athletic activity, psychosocial connection to the hemophilia patient community, and patient satisfaction have not been assessed. Additionally, reasons for choosing to initiate or not initiate emicizumab in patients who have been offered it have not been evaluated. This patient survey study addresses these questions.
Methods: A voluntary, anonymous 22-item survey was electronically distributed to hemophilia patients throughout the United States through hemophilia treatment centers and the National Hemophilia Foundation. Recipients were instructed to take the survey only if they were a patient with hemophilia A who has been offered bleeding prophylaxis (or the parent of such a patient under age 18). Skip logic was employed such that individual respondents answered 15 or fewer items based on their familiarity with and use of emicizumab. Results to items utilizing a 5-point Likert scale were converted to weighted average scores (ranging from 1, lowest to 5, highest) for comparison between groups of respondents.
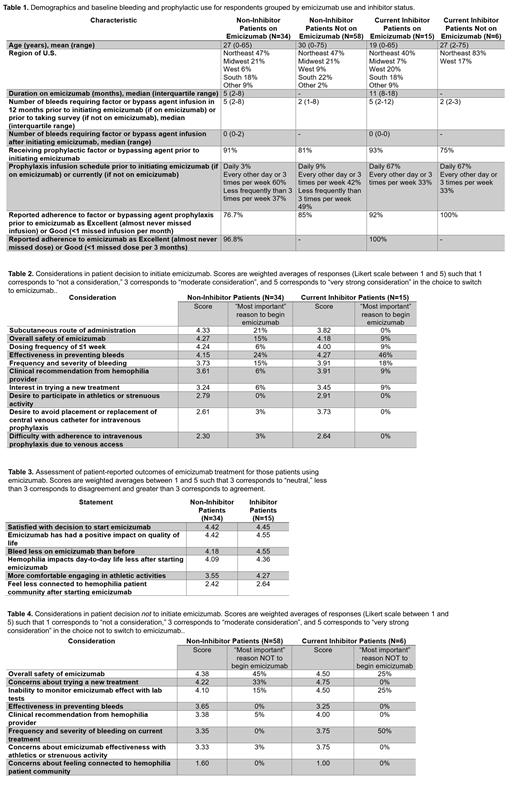
Results: 126 respondents (67 adults and 59 parents of children age <18) answered the survey, 82% without an inhibitor and 18% who currently have an inhibitor (Table 1). 89% of respondents were familiar with emicizumab, of whom 43% were using and 57% had never used emicizumab. Table 1 describes respondent demographics and baseline bleeding and prophylaxis characteristics by emicizumab use and inhibitor status. Patients using emicizumab had higher reported median annualized bleeding rates (ABR) prior to emicizumab than those not using emicizumab regardless of inhibitor status (5 bleeds/year vs. 2 bleeds/year) and patients using emicizumab reported lower adherence with prior factor prophylaxis than current emicizumab prophylaxis (76.7% vs. 96.8 described adherence as good or excellent). Non-inhibitor patients emphasized subcutaneous administration [score 4.33, 21% cited as most important reason (MIR) for initiating emicizumab] and ability to dose ≤1 time per week (4.24, 6%) as equally or more important than safety (4.27, 15%) and effectiveness in bleed prevention (4.15, 24%), Table 2. By contrast, the top concern of inhibitor patients was effectiveness in bleed prevention (4.27, 46%) and safety (4.18, 9%), with lifestyle concerns such as subcutaneous administration (3.82, 0%) less important. In consideration of the impact of emicizumab on wellbeing, most patients were highly satisfied with their decision to start emicizumab (Table 3). Emicizumab use did not result in perceived distancing from the hemophilia patient community. Non-inhibitor patients deciding not to initiate emicizumab (Table 4) cited safety concerns (score 4.38, MIR 45%), unknowns of a new treatment (4.22, 33%), and lack of lab monitoring of emicizumab effect (4.10, 15%) as the primary reasons to forego emicizumab; effectiveness (3.35, 0%) was not a major concern. Of the 64 respondents not using emicizumab, 60% had discussed it with their hemophilia doctor; of these patients, 90% chose to wait for more experience with its use and 10% would have started it but could not due to insurance denial. Of the 40% who had not discussed emicizumab with their hemophilia doctor, 67% felt they learned enough about emicizumab from patient groups or marketing and had decided not to begin the agent; 33% felt they did not have enough information to make a decision about using it.
Conclusions: Most U.S. hemophilia A patients requiring prophylaxis are familiar with emicizumab. Regardless of inhibitor status, patients with higher ABR and worse compliance with infusion-based prophylaxis are more likely to use emicizumab. From the non-inhibitor patient perspective, ease of administration with subcutaneous dosing ≤1 time per week was at least as important as effectiveness and safety of the drug in their decision to switch to emicizumab, whereas this was much less important to inhibitor patients. Patients using emicizumab were highly satisfied with their decision to switch to this agent. Patients choosing not to switch to emicizumab are concerned about safety, unknowns with a new treatment, and lack of lab monitoring, although one-third of patients felt they lacked the information to decide whether to use it.
Al-Samkari:Dova: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Moderna: Consultancy. Croteau:Octapharma: Honoraria; Bayer: Consultancy, Honoraria; Pfizer: Research Funding; Genentech: Consultancy, Honoraria; Spark Therapeutics: Research Funding; CSL Behring: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal