
Background: Emerging data suggest fixed four-factor prothrombin complex concentrate (4F-PCC) dosing may adequately reverse warfarin, minimize time to INR reversal, and reduce medication costs. A 4F-PCC fixed-dosing strategy for INR reversal was implemented at The Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center. We describe the outcomes associated with implementation of this dosing protocol, and compare outcomes among patients receiving two 4F-PCC fixed-dosing strategies.
Methods: According to the 4F-PCC fixed-dosing protocol, patients with warfarin-induced intracranial hemorrhage (ICH) with actual body weight > 50 kg received Kcentra® 1,500 units once, while patients < 50 kg received Kcentra® 1,000 units once. Regardless of the initial dose, all warfarin-induced ICH patients with inadequate INR reversal after initial dosing qualified for repeat dosing with Kcentra® 500-1,000 units if clinically indicated. Patients requiring INR reversal for non-ICH indications received Kcentra® 1,000 units once, with a repeat dose of Kcentra® 1,000 units administered for inadequate INR reversal if clinically indicated. Patients who received 4F-PCC fixed-dosing from January 18, 2019 to June 18, 2019 were included. Patients who received Kcentra® doses > 1,700 units due to protocol non-adherence were excluded. Patients were included in the efficacy analysis if they had a baseline INR > 1.4 and a post-Kcentra® administration INR result was available. All patients receiving fixed 4F-PCC during this time period were included in the cost savings analysis, regardless of baseline INR.
Results: A total of 51 patients received 52 administrations of fixed-dose Kcentra® during the study period. Six patients received FDA-approved Kcentra® dosing, and were excluded. Kcentra® 1,000 units was administered for non-ICH INR reversal indications in 25 (48%) cases. Kcentra® 1,500 units was administered for INR reversal in the setting of warfarin-induced ICH in 19 (36.5%) cases. Fixed Kcentra® dosing deviated from our institutional dosing protocol in eight of 52 cases. The most common indications for Kcentra® administration were ICH (36.5%), refractory hemorrhage after cardiac surgery (17%), gastrointestinal bleeding (11.5%), and emergent procedure or surgery (7.7%).
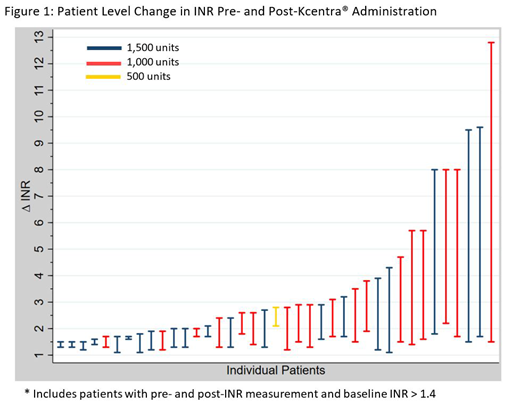
For patients in the efficacy analysis (n=39), the median baseline INR was 2.8 (IQR 1.95-4.1), the median time to INR measurement after Kcentra® administration was 83 minutes (IQR 40-173), and the median weight was 70.3 kg (IQR 61.8-92.2). Thirty-two patients (82.1%) in the efficacy analysis received an intravenous dose of vitamin K 10 mg in addition to Kcentra®, and two patients received an intravenous dose of vitamin K 5 mg. The change in INR before and after Kcentra® administration is shown in Figure 1. Thirty-seven (94.8%) patients included in the efficacy analysis achieved an INR measurement < 2 following Kcentra® administration. Twenty-eight (71.7%) of patients included in the efficacy analysis achieved an INR of < 1.7 following Kcentra® administration. One patient required repeat Kcentra® dosing.
When comparing patients who received Kcentra® 1,000 units to 1,500 units, there was no significant difference in achievement of INR values < 2.0 (94.4% vs. 95.2%, p=0.72) or < 1.7 (66.7% vs. 76.2%, p=0.51). Additionally, there was no significant difference in achievement rates of INR values < 1.7 in those with baseline INRs > 4.0 in the Kcentra® 1,000 unit group compared to 1,500 units (66.7% vs. 40%, p=0.392). Utilizing the actual wholesale price of 2.90 USD per unit, the estimated cost savings from use of Kcentra® fixed-dosing was 148,348 USD.
Conclusions: Administration of Kcentra® 1,000 to 1,500 units effectively reduced the baseline INR to < 2 in 94% of cases. There were no significant differences in achievement of post-treatment INR values of <2.0 or < 1.7 between those who received Kcentra® 1,000 units versus 1,500 units. Achievement rates of INRs < 1.7 after Kcentra® administration in patients with significantly elevated baseline INRs (INR > 4) were also not significantly different between treatment groups. However, these findings must be confirmed in larger studies due to the small sample size in this analysis. Additionally, implementation of a 4F-PCC fixed-dosing protocol is associated with significant cost savings.
Shanbhag:Daiichi Sankyo/Lultpold Pharmaceuticals: Research Funding. Streiff:Pfizer: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Portola: Consultancy, Honoraria; Roche: Research Funding; Daiichi-Sankyo: Consultancy, Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Kcentra: used in some patients in our cohort for reversal of non-warfarin-associated INR elevation
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal