Introduction: 8500 adult allogeneic hematopoietic stem cell transplants (alloHSCT) are performed annually in the U.S. and 17,000 in Europe. HSCT-associated thrombotic microangiopathy (TMA), defined by thrombocytopenia, microangiopathic hemolytic anemia, and organ dysfunction in the absence of disseminated intravascular coagulation, complicates some 20% of these procedures. About half of post-alloHSCT TMAs may resolve when GvHD immunoprophylaxis is modified, but three year survival rates for those with severe HSCT-TMAs are a dismal 11%. Clinical manifestations are similar to other TMAs, but their pathophysiology may be distinct. Damage to vascular endothelium, independent of loss of ADAMTS13 activity, is thought to be critical. Fibrin-rich microthrombi, often accompanied by C4d and C5b-9 (membrane attack complex) deposition, occur in the microvasculature of multiple organs (Clin Adv Hematol Oncol 2014;12:565-573; Transplantation. 2013;96:217-223). This supports a role for complement activation in HSCT-TMA, but the importance of the various complement activation pathways is unclear. Recently, narsoplimab (OMS721), a monoclonal antibody inhibitor of MBL-associated serine protease-2 (MASP-2), a principal component of lectin-dependent complement activation, received Breakthrough Therapy Designation for HSCT-TMA, based on improved survival compared to historical controls in a phase 2 study. A phase 3 program is ongoing. Chemotherapy in association with autologous HSCT is linked to a marked increase in serum MASP-2 levels, persisting for about 4 weeks post-transplant (Front Immunol 2018;9:2153). However, TMAs are rare in autologous transplants, and circulating levels of MASP-2 following alloHSCT, and the impact of TMA development on those levels, are unknown.
Methods: All individuals >18 years of age scheduled to undergo alloHSCT for hematologic malignancy at New York Presbyterian Hospital-Cornell were approached to participate in this study (NCT02604420). 100 of the first 101 subjects, age 58.3 +14 yrs, were enrolled and followed for >1.5 years post-transplant. This interval is consistent with the median time to HSCT-TMA diagnosis in adults of 90 days (range 32-733 days). Plasma was obtained at baseline (defined as between the time of consent and beginning of conditioning regimen), and at each regularly scheduled visit post-transplant-day 28 +5 days; day 100 +28 days; day 180 +28 days; and day 365 +28 days-as well as at the time of TMA diagnosis, based on the Consensus Criteria of Cho et al. (Transplantation 2010;90:918-926). A commercial ELISA (MyBioSource) was used to assess MASP-2 concentrations.
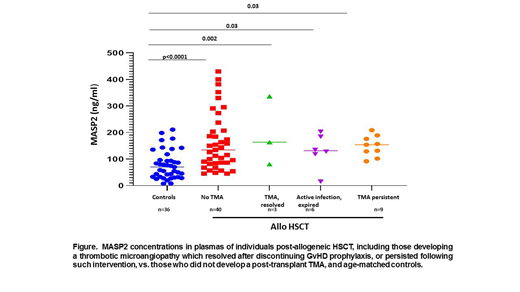
Results: 20 subjects met study criteria for a HSCT-TMA diagnosis, occurring a median of 69 days (range 33-289) post-transplant. Three resolved following discontinuation of GvHD prophylaxis (mTOR or calcineurin inhibitor) and switch to mycophenolate and increased corticosteroid doses, and 7 had an intercurrent infection, 6 of whom expired with ongoing severe TMA despite a change in GvHD prophylaxis (Figure). TMAs persisted in the remaining 10 subjects. Median MASP-2 levels were significantly elevated in all subjects post-transplant, assessed at the time of TMA development or, in those not developing a TMA, at day 100 + 28 days post-transplant vs. controls (n=36, 86.2ng/ml (23.3-210.9): persistent TMA (n=9 (one plasma unavailable), 154ng/ml (range 82-209)); alloHSCT subjects who did not experience a TMA (n=40 evaluated to date, 113.5ng/ml (56-430.3)). (Figure). Lack of a significant rise in MASP-2 levels in patients with persistent TMAs vs. those who did not develop a TMA, combined with a significant decrease in variance of MASP-2 levels in the former group (p=0.005), may reflect consumption of product at sites of disease activity, i.e., the microvasculature.
Conclusions: There is a significant increase in MASP-2 levels, with a wide variance, in post-alloHSCT patients evaluated at a time post-transplant typical of HSCT-TMA development. At time of development of a HSCT-TMA that persists despite withdrawal of GvHD prophylaxis, MASP-2 levels remain elevated over controls, but with a significantly lower variance vs. those not developing TMA. A study of additional samples, including longitudinal specimens, from this cohort is underway to determine if a change in MASP-2 levels correlates with HSCT-TMA development post-alloHSCT.
Van Besien:Miltenyi Biotec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal